What Do You Know About Lyme Disease Prevention
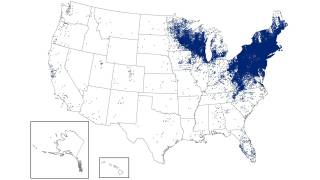
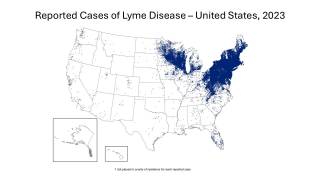
While the 2025 Lyme disease (LD) season has come to a close in most of the United States, it has expanded significantly since its discovery in Connecticut decades ago.
Lyme disease has become the most common vector-borne disease in the USA, with an estimated 476,000 cases diagnosed and treated each year.
A recent online survey conducted in 28 states by researchers from Pfizer Inc. and the Johns Hopkins Bloomberg School of Public Health found that a majority of respondents were aware of LD. Still, many were not taking steps to prevent it.
The survey, published by the Open Forum Infectious Diseases on October 21, 2025, also revealed that a significant number of adults (88%-92%) and children (96%-98%) spend time in their yards.
Additionally, 70% to 79% of adults and 83% to 84% of children participate in recreational outdoor activities.
However, only 34-40% of adults and 40-42% of children always used preventive measures during outdoor recreational activities.
These researchers wrote, 'Although most people in high-incidence areas engaged in outdoor activities at least occasionally, few reported being at high or very high risk of LD. It is possible that people do not fully understand the types of outdoor activities that put one at risk of LD.'
'For example, most tick encounters are reported from daily activities like walking a dog or spending time in a yard, and fewer after activities like hiking or camping.'
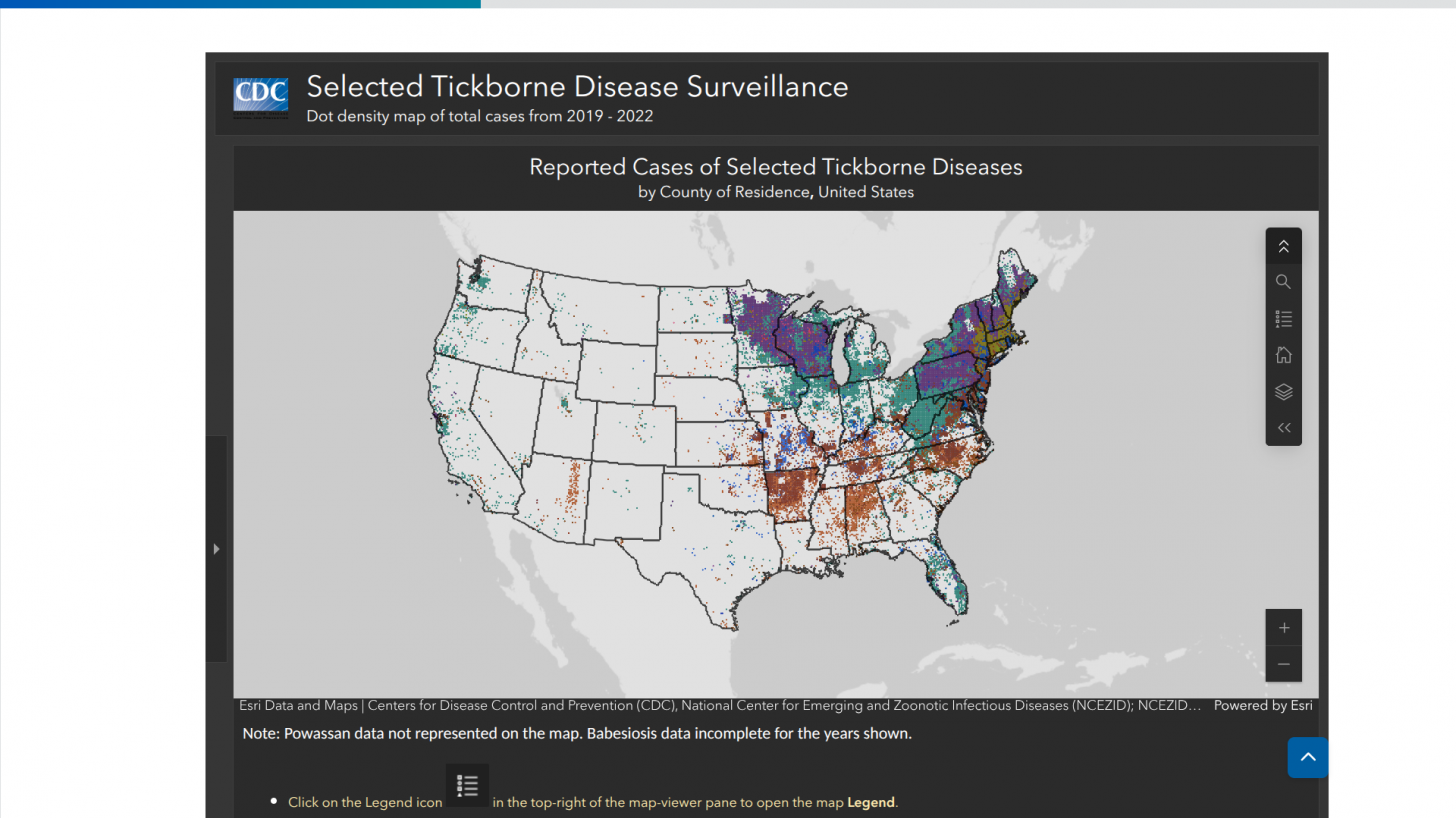
Furthermore, as the highlight map indicates, LD is not the only Tickborne disease spreading in the USA.
Once infected, early diagnosis and appropriate antibiotic treatment can help prevent more severe diseases such as LD, according to the U.S. CDC.
In general, the U.S. CDC does not recommend antibiotics after tick bites to prevent Tickborne diseases. The antibiotics most commonly used to treat Lyme disease include doxycycline, amoxicillin, or cefuroxime axetil.
From a disease prevention perspective, an innovative vaccine candidate co-developed by Pfizer is making steady progress in clinical trials.
The U.S. Food and Drug Administration granted Fast Track designation to the VLA15 vaccine development program in July 2017. As of November 3, 2025, VLA15 is the only Lyme disease vaccine candidate in advanced phase 3 clinical development.
Furthermore, there is growing anticipation from nature seekers for this vaccine's approval in 2026.
Our Trust Standards: Medical Advisory Committee