Future Lyme Disease Test Could Be 90% Accurate
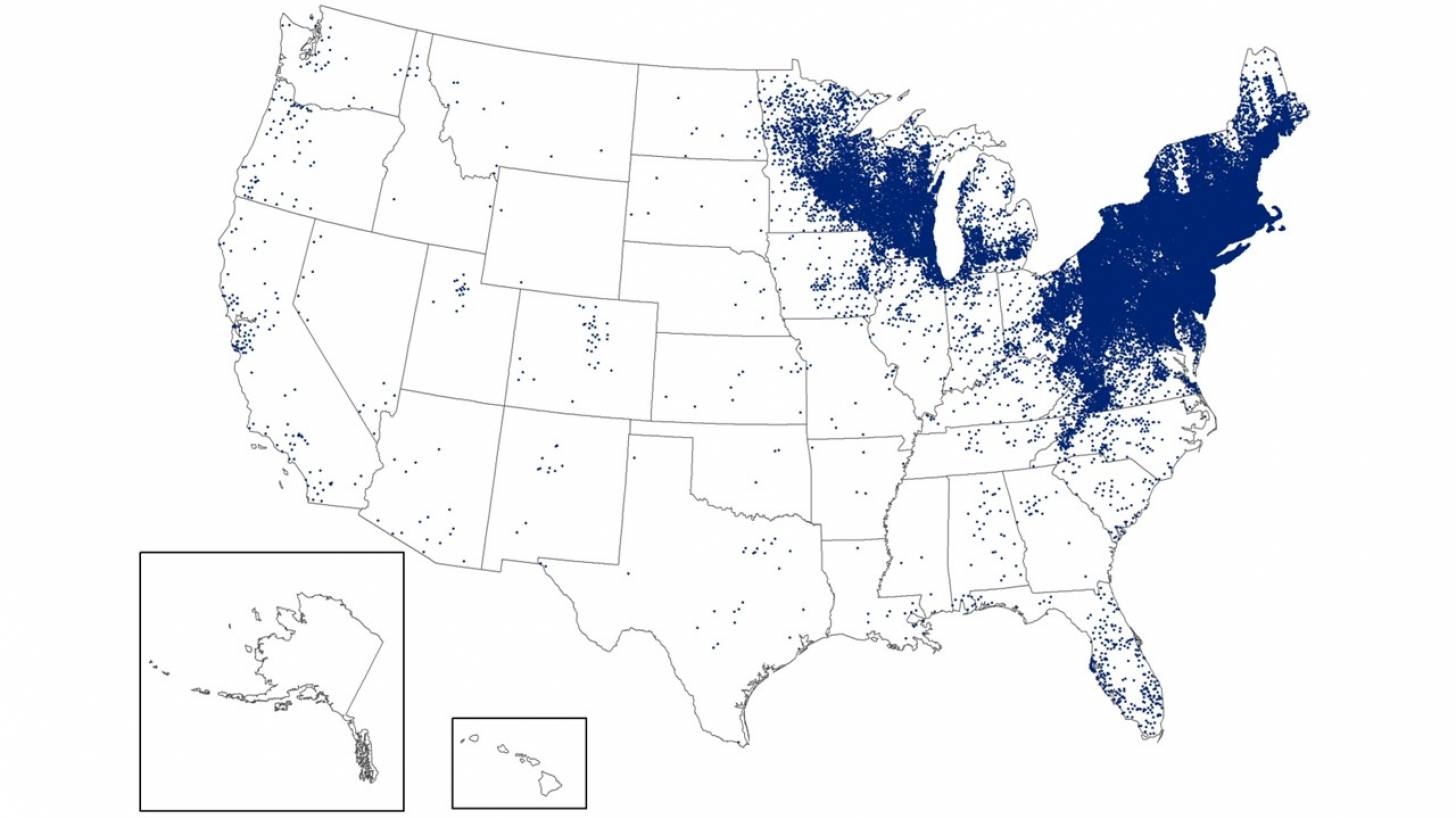
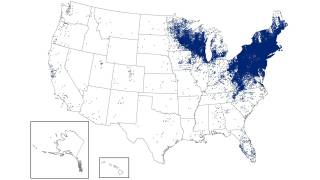
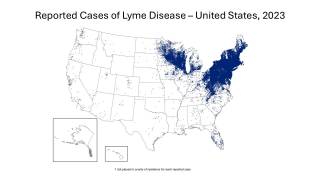
Over the past 30 years, Lyme disease, which was initially detected in Connecticut, has been on the rise, particularly in the Northeast and recently the upper Midwest regions. These increase is linked to ticks carrying the bacterium Borrelia burgdorferi.
While the characteristic bull's-eye rash is an early sign of the disease, it only occurs in about 25% of patients. Many patients develop skin lesions that mimic other illnesses, complicating the clinical diagnosis.
Additionally, antibody tests often yield false negatives during the first few weeks of infection.
To address these diagnostic challenges, researchers at Dartmouth Hitchcock Medical Center have developed a new method to detect Borrelia burgdorferi, enabling faster, more accurate diagnoses.
Guohong (Grace) Huang, Ph.D., led the project that began with a case involving a 73-year-old woman who experienced progressively worsening skin hardening and inflammation, as well as joint immobility over four years. Initially, doctors believed she had morphea, a condition that causes hard, thickened patches of skin.
However, the immunosuppressant medication used to treat morphea did not improve her symptoms. In this case, antibody testing showed only evidence of a past Lyme infection, but the patient responded to doxycycline, an antibiotic commonly used to treat Lyme disease.
Shaofeng Yan, M.D., Ph.D., a member of her care team, requested that Huang's department develop a new molecular test to confirm the presence of Borrelia burgdorferi. The team created three droplet digital PCR (ddPCR) tests that accurately and sensitively measure different types of Borrelia bacteria.
One test detects all Borrelia species, another targets those responsible for Lyme disease, and a third is specific to Borrelia burgdorferi, the leading cause of Lyme disease in the U.S.
When tested on a limited number of skin samples from patients with confirmed or suspected Lyme disease, the new assays showed excellent accuracy. They could detect as few as five to ten bacterial cells.
Ultimately, the test for Borrelia burgdorferi developed by the team had an estimated sensitivity of 90.9% in formalin-fixed, paraffin-embedded samples, making it a promising new tool for Lyme disease testing.
Sensitivity is expected to improve when using fresh or frozen tissues, which typically yield DNA of higher quantity and quality.
"Using the ddPCR assay, we successfully detected B. burgdorferi DNA in this patient's skin biopsy," Huang commented in a press release on November 14, 2025.
"This finding was further confirmed by DNA sequencing, supporting the diagnosis of chronic Lyme disease." Huang also noted that traditional serological tests cannot distinguish between active infection and past exposure."
"Antibody levels may remain elevated even after successful treatment. This is another clinical scenario where the ddPCR assay offers a clear advantage by detecting active bacterial presence rather than relying on indirect immune markers," Huang added.
Although it took the patient more than four years to get a final diagnosis, the test developed by Huang and her team could save future patients from such extended waits. Early diagnosis is crucial in reducing the risk of long-term complications. With further development of the team's test, prompt treatment will be possible for more patients.
"To advance this work, the next step is to expand testing to a large number of cases and explore strategies to enhance the assay sensitivity further," Huang said.
With this diagnostic test and a preventive Lyme disease vaccine candidate (VLA15) becoming available in the future, this Tickborne disease's impact on outdoor activities may soon dissipate.
Our Trust Standards: Medical Advisory Committee