Lyme Disease Vaccine Needed More Than Ever

Spread by infected ticks, Lyme disease is a bacteria notoriously difficult for people to identify.
While some people know to look out for a circular rash after walking in the woods, many overlook this quiet disease. Others display symptoms that could be easily mistaken for the flu.
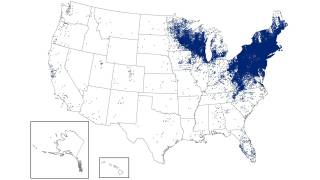
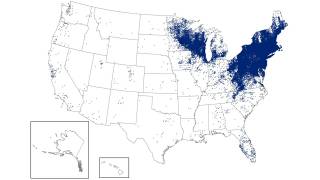
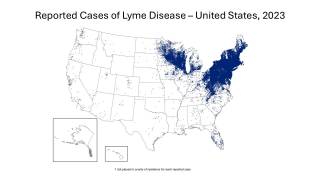
Though Lyme disease (Borrelia burgdorferi) cases have been reported in most states, the data can be misleading.
Most Lyme disease cases are reported where the person lives, not the actual location where they were infected.
Many health officials believe a Lyme vaccine is needed now more than ever.
Lyme disease is considered the most widespread disease transmitted by insects in the U.S. According to one study by the Centers for Disease Control and Prevention (CDC), the number of counties in the USA reporting Lyme has nearly quadrupled in 20 years.
Moreover, with an estimated 240,000 to 440,000 new cases diagnosed every year, researchers have found that Lyme disease costs the U.S. healthcare system between $712 million and $1.3 billion a year.
Which means, treating Lyme disease costs on average $3,000 per patient.
But, no Lyme borreliosis vaccine is currently available for humans, although it has been shown that the disease can be prevented by immunization with an Outer Surface Protein A-based vaccine.
Back in February 2002, just over three years after receiving FDA approval, GlaxoSmithKline decided to withdraw its Lyme disease vaccine LYMErix from the market.
CDC researchers concluded the LYMErix vaccine was 80 percent effective, but a public relations fiasco.
Since then, people have been left without a viable preventive solution.
That may be why Valneva, a European biotech company, recenty received Fast Track status from the FDA for the development of its Lyme vaccine, VLA15.
VLA15 is a new hexavalent, protein subunit-based vaccine candidate targeting the Outer Surface Protein A of Borrelia, the most dominant protein expressed by the bacteria when present in a tick.
The first human trials are underway in the United States and Europe. As such, Valneva plans to initiate Phase II as early as the first quarter of 2018.
What’s preventing Valneva’s VLA15 vaccine from succumbing to the same pitfalls as LYMErix?
Previous research found the primary issues that led to the LYMErix withdrawal appear to be a combination of vaccine safety concerns, the vaccine cost and a difficult vaccination schedule.
Until the FDA approves a new vaccine for use, the CDC suggests people use repellent that contains 20 percent or more DEET, picaridin, or IR3535 on exposed skin for protection. Pre-treating clothing is recommended and may offer longer protection.
Our Trust Standards: Medical Advisory Committee
- VALNEVA Receives FDA Fast Track Designation for its Lyme Disease Vaccine Candidate VLA15
- Lyme Disease Rashes and Look-alikes
- Signs and Symptoms of Untreated Lyme Disease
- Data and Statistics
- Geographic Distribution and Expansion of Human Lyme Disease, United States
- Vaccines against Lyme Disease: What Happened and What Lessons Can We Learn
- New Anti-Lyme Disease Treatment Being Tested, But Could Face Troubles Ahead
- Design and Development of a Novel Vaccine for Protection against Lyme Borreliosis