Lyme Disease Patients to Benefit from New Diagnostic and Vaccine Efforts in 2026
The U.S. Department of Health and Human Services (HHS) held a roundtable discussion today with Lyme disease patients, clinicians, and researchers focusing on diagnostics and clinical needs related to this tick-borne illness.
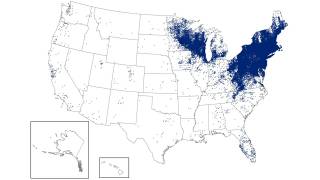
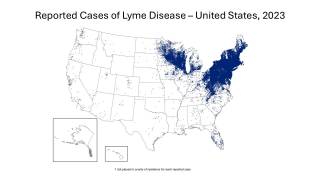
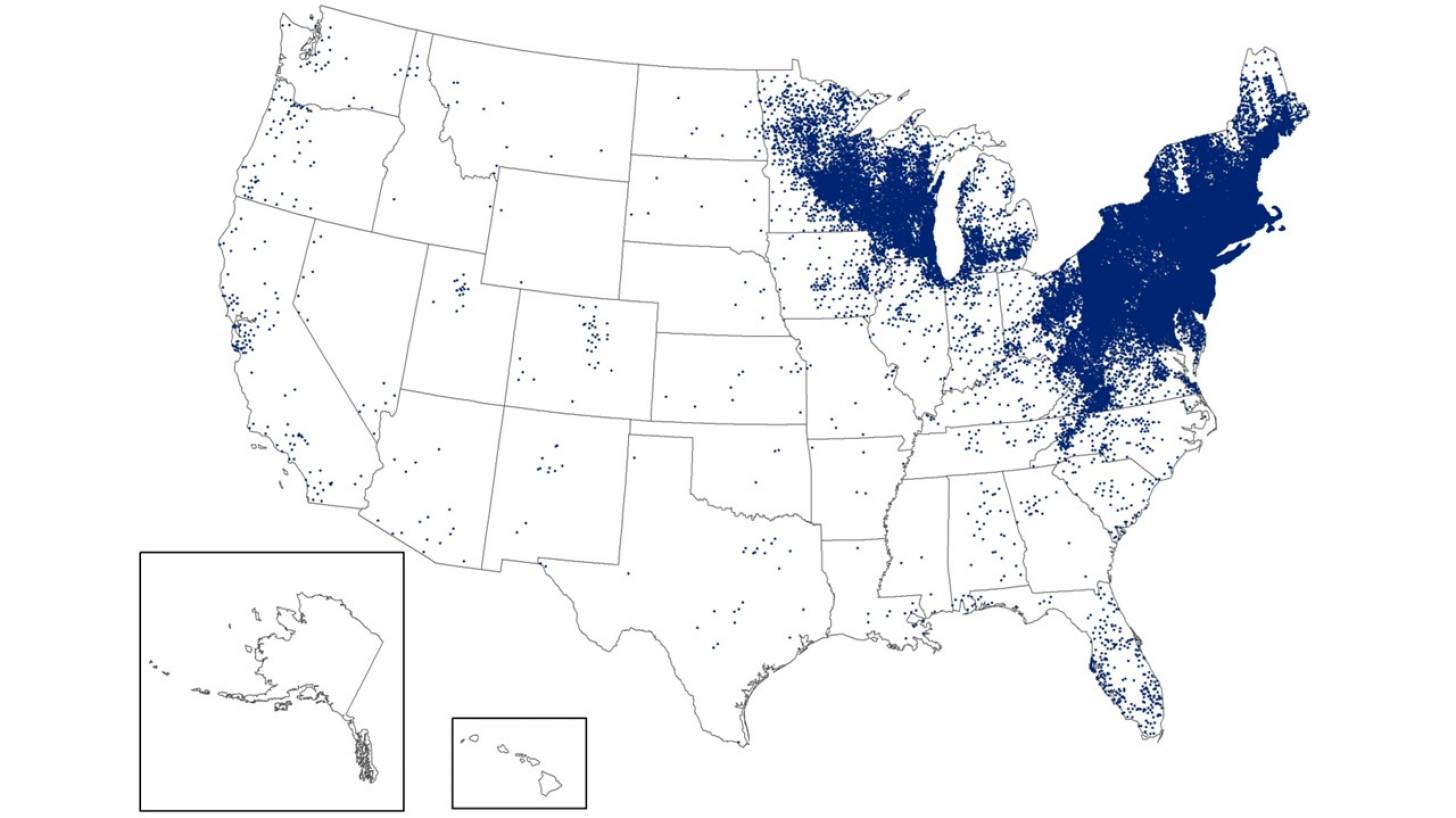
According to data from the U.S. Centers for Disease Control and Prevention (CDC), since its identification in the late 1960s, Lyme has infected an estimated 5 to 7 million Americans over the past decade, many in the Northeast and Midwest of the USA.
Furthermore, the CDC estimates that for up to 20% of patients, symptoms persist and progress into Lyme infection-associated chronic conditions and illnesses. Many of these patients are undiagnosed for Lyme infection.
As part of the HHS event on December 15, 2025, HHS announced the renewal of the LymeX Innovation Accelerator. Established in 2020, LymeX is the most significant public-private partnership ever built to improve Lyme disease diagnostics and care.
The updated LymeX effort will expand patient-centered innovation and next-generation diagnostics. AI-enabled methods and high-dimensional biological tools are improving the understanding of persistent symptoms caused by Lyme bacteria.
LymeX also encompasses the LymeX Diagnostics Prize, with over $10 million in cash prizes underwritten by the Steven & Alexandra Cohen Foundation, to advance diagnostic tools that support earlier and more accurate detection across stages of the infection.
Currently, research teams are in Phase 4, progressing innovative diagnostics through clinical validation and regulatory pathways.
HHS leadership stated it will advance this work through a national strategy based on open data, transparent research practices, and direct engagement with patients.
HHS also announced that the Centers for Medicare & Medicaid Services has provided guidance clarifying that it supports beneficiaries with Lyme disease and related conditions through the Chronic Care Management program. This support expands access to coordinated care and reduces financial barriers for Medicare patients with complex, long-term conditions.
Seperately, two pharmaceutical firms partnered in 2020 to develop a Lyme Disease vaccine.
France-based Valneva SE, in partnership with Pfizer Inc., is developing VLA15, a Lyme disease vaccine candidate targeting the outer surface protein A of the Borrelia bacteria, aiming to block transmission from ticks to humans by generating antibodies that prevent the bacteria from spreading within the tick.
On November 26, 2025, Valneva announced positive final immunogenicity and safety data from the Phase 2 study, VLA15-221.
The study's results showed a strong anamnestic immune response and a favorable safety profile six months after a third booster dose (month 48) in all age groups, confirming compatibility with the anticipated benefits of a yearly vaccination before each Lyme season.
Currently, phase 3 studies cover six prevalent serotypes in North America and Europe. VLA15 has shown positive safety and immune response data in adults, adolescents, and children, with potential regulatory filings planned for 2026, if research results remain positive.
Our Trust Standards: Medical Advisory Committee