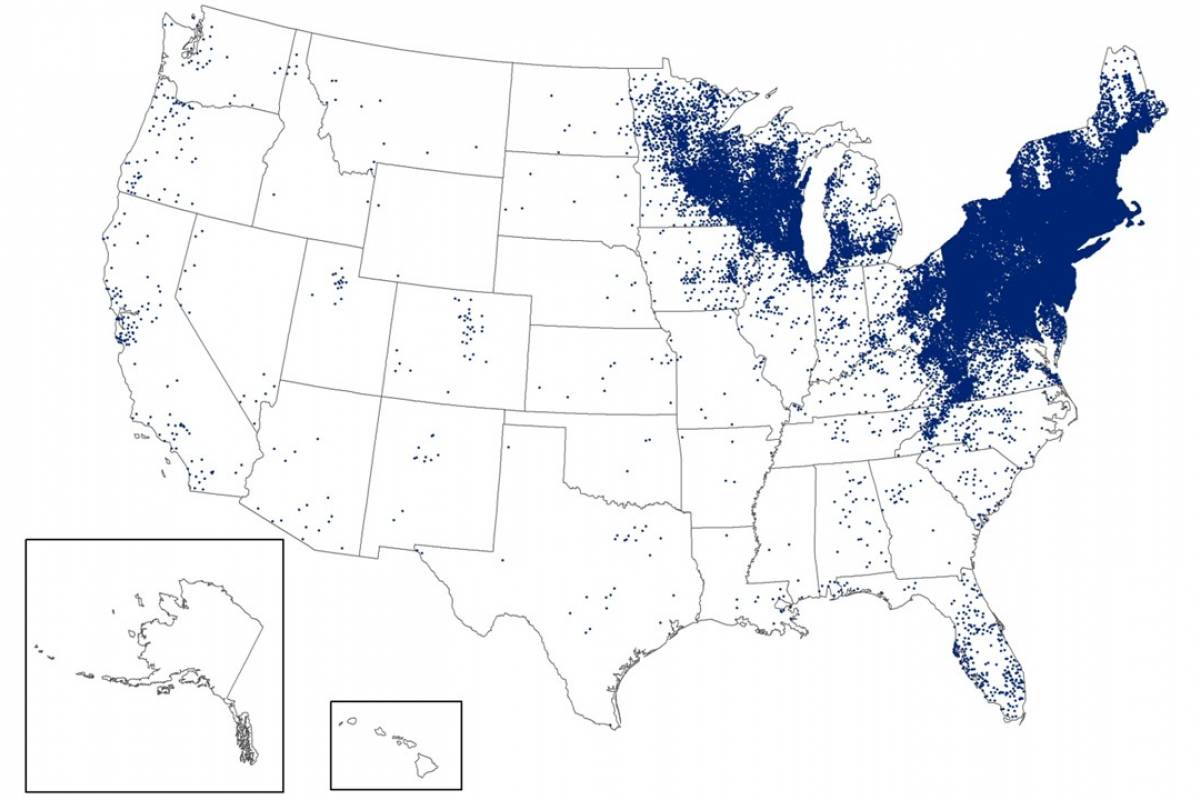
Lyme Disease Vaccine Candidate Is On-Track
Valneva SE today reaffirmed that the Phase 3 clinical trial of its Lyme disease vaccine candidate, VLA15, remains on track.
The company's press release on October 6, 2025, states that Participants in the VALOR clinical trial will be monitored for the occurrence of Lyme disease cases until the end of 2025. Valneva expects the VALOR trial outcomes to be announced in the first half of 2026, followed by planned regulatory submissions.
Valneva's development partner, Pfizer Inc., continues to aim to submit a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) and a Marketing Authorization Application to the European Medicines Agency in 2026, pending the receipt of positive Phase 3 data.
Pending approval, Valneva expects Pfizer to launch the vaccine in the second half of 2027.
The FDA granted the VLA15 vaccine development program Fast Track designation in July 2017, and it remains the leading candidate in development.
The VLA15 vaccine protects humans by raising antibodies that prevent Borrelia from migrating from ticks after a bite. VLA15 is designed to cover about 97% of Borrelia in North America and Europe. VLA15 is being tested as an alum-adjuvanted formulation and administered intramuscularly.
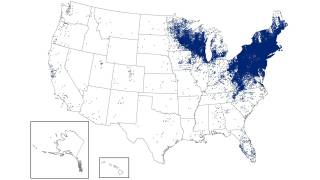
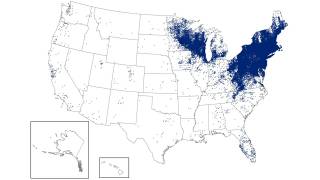
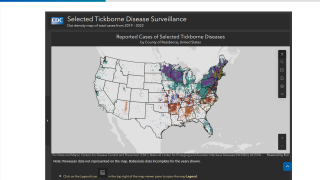
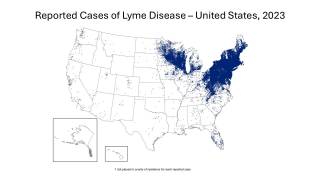
While Lyme disease has been found in the northeastern USA for decades, ticks in the upper Midwest are now spreading this severe disease.
Our Trust Standards: Medical Advisory Committee