Should Florida Authorize a Second-Generation Dengue Vaccine
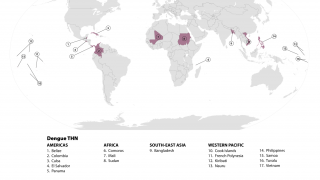
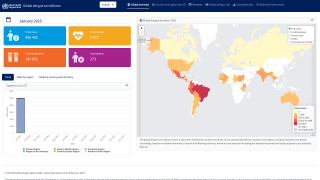
In response to the growing threat of dengue fever in the Americas, the Pan American Health Organization (PAHO) has been actively working to control the spread of this mosquito-borne disease since 2003.
Currently, 46 countries and territories in the region are reporting dengue cases to the PAHO.
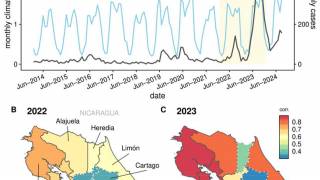
Last year, there was an unprecedented surge in cases, with over 13 million reported dengue infections and 8,431 related fatalities across the Americas.
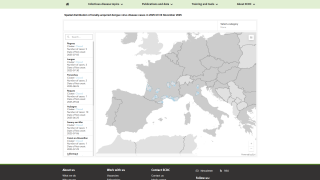
As of November 11, 2025, the PAHO has confirmed more than 4.1 million dengue cases and 2,041 associated deaths.
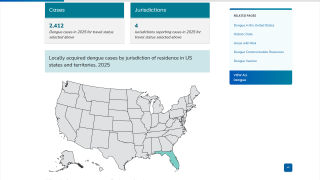
And in the United States, the U.S. CDC reported in late September 2025 that 2,560 locally acquired dengue cases had been documented across four jurisdictions this year.
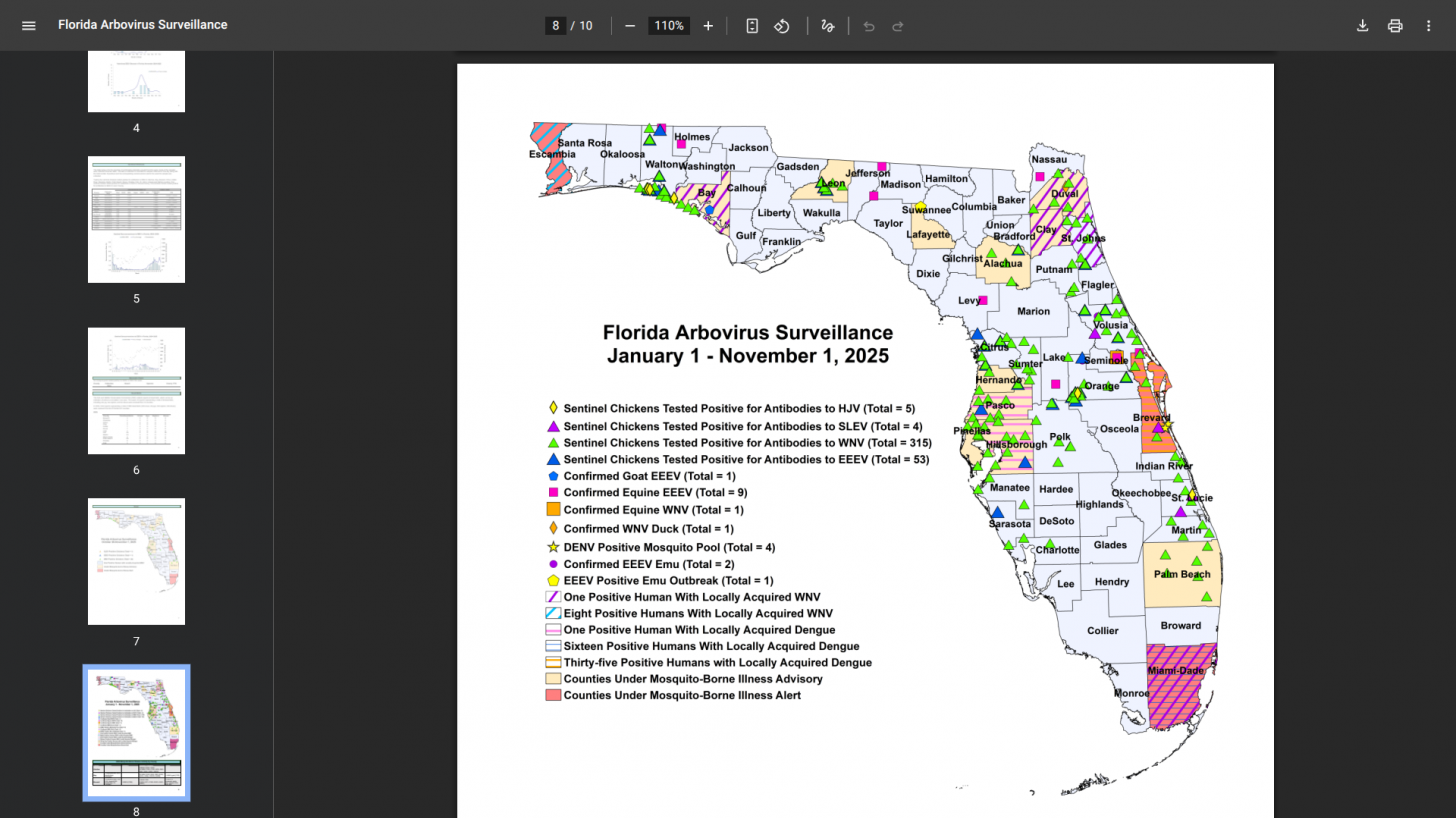
Traditionally, Miami-Dade County, Florida, has reported the most local dengue cases.
However, during 2025, Brevard County (35) has surpassed Miami-Dade (16),
Given the 180-mile distance along Florida's east coast between these counties, there could be many undetected cases of dengue.
Regarding disease prevention, the CDC states that the first-generation Dengvaxia vaccine is currently the only one available in the U.S.
The CDC, in collaboration with the Puerto Rico Department of Health, is evaluating Dengvaxia in children, despite the manufacturer having discontinued production. These health agencies assert that Dengvaxia is safe and effective when administered as recommended.
Other dengue vaccines have either been approved or are in late-stage development; however, they are not currently available in the U.S.
The global leader is Takeda Pharmaceuticals' second-generation QDENGA® vaccine, currently authorized in 41 countries, where over 18 million doses have been distributed in endemic countries.
On November 3, 2025, Takeda announced data, including an exploratory analysis of a booster dose, confirming the favorable benefit-risk profile of QDENGA and that the two-dose regimen provides sustained protection against dengue over 7 years.
Derek Wallace, President of the Global Vaccine Business Unit at Takeda, informed Vax-Before-Travel (VBT) on November 11, 2025, "Dengue is spreading to areas once considered low-risk, with year-round peak seasons in many regions."
"These long-term data show that QDENGA's two-dose schedule provides sustained protection without a booster, simplifying vaccination efforts and improving our ability to combat dengue globally."
Since the CDC's vaccine committee appears not to be taking action to approve QDENGA in 2025, should Joseph A. Ladapo, MD, PhD, Florida's Surgeon General, pave his own path and approve QDENGA?
Ladapo has already provided distinct guidance regarding COVID-19 and measles vaccinations this year.
Don Hackett, VBT's publisher, asks.... as winter vacations are being planned for dengue-outbreak destinations in the Americas, isn't it good public health policy to ensure that international travelers have an option in 2025 to protect themselves against this serious disease?
Our Trust Standards: Medical Advisory Committee