Second-Generation Dengue Vaccine Delivers Seven Years of Protection

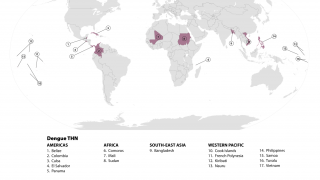
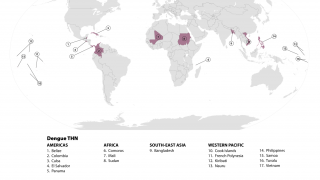
Since its first approval in Indonesia in 2022, Japan-based Takeda's QDENGA® dengue vaccine has been authorized in 41 countries and distributed in 11 dengue-endemic countries to help reduce the global threat posed by this mosquito-transmitted disease.
Today, Takeda announced very positive vaccine efficacy data.
On November 3, 2025, the company announced the completion of the 7-year pivotal Phase 3 Tetravalent Immunization against Dengue Efficacy Study (TIDES) trial evaluating QDENGA. These data, including an exploratory analysis of a booster dose, confirm the favorable benefit-to-risk profile of QDENGA and that the two-dose regimen provides sustained protection against dengue.
This data is consistent with its approved indications in multiple countries worldwide, which could simplify vaccination schedules and increase adherence.
"QDENGA is the most comprehensively studied dengue vaccine, with more than 60,000 participants globally in the clinical program, and these long-term data highlight the durability of its safety and efficacy profile, across diverse populations worldwide," commented Derek Wallace, M.D., president of the Global Vaccine Business Unit at Takeda, in a press release.
"We are proud to have worked hand-in-hand with the clinical trial participants, collaborators, and investigators whose contributions have been integral to the success of the TIDES trial and played a role in helping us move closer to a dengue-free world."
Takeda stated it continues to invest in post-marketing research through real-world evidence generation and ongoing pharmacovigilance to deepen understanding of the vaccine's safety and impact.
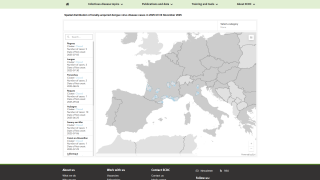
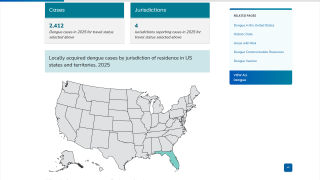
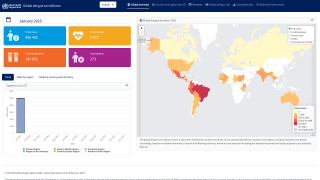
Obtaining access to this market-leading vaccine is essential for states such as Florida, which has reported 53 locally acquired dengue cases in Brevard, Hillsborough, Miami-Dade, and Pasco counties in 2025.
As of today, QDENGA is unavailable in the United States.
Our Trust Standards: Medical Advisory Committee