Unintentional Dengue Vaccination Produce Few Side Effects for Neonates

Millions of couples vacation in the Caribbean, where Dengue virus is endemic. While visiting these countries, some women have gotten vaccinated with the second-generation Dengue vaccine, which is not an authorized use.
TAK-003 (QDENGA) vaccination is authorized in about 40 countries in 2025 but is contraindicated during pregnancy and lactation.
Original Research published by Taylor and Francis Online on March 18, 2025, found no evidence of increased adverse pregnancy outcomes following unintentional TAK-003 vaccination occurring inside the exposure window compared with placebo.
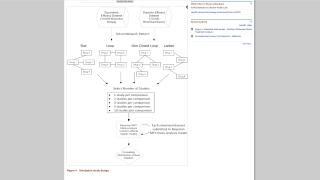
This post-hoc analysis of phase 2 and 3 clinical studies evaluated pregnancy outcomes and neonatal adverse events (AEs) following unintentional vaccination when women could be pregnant within 44 days before the last menstrual period until the outcome of pregnancy.
Of the 557 reported pregnancies in these studies, 38 occurred inside the exposure window. Of these, 28 (TAK-003, n = 23; placebo, n = 5) resulted in live births, four resulted in elective terminations (TAK-003, n = 2; placebo, n = 2), five in spontaneous abortions (TAK-003, n = 3; placebo, n = 2) and one unknown outcome (placebo).
Of the spontaneous abortions, there was no significant difference between TAK-003 and placebo recipients or between those occurring within or outside the exposure window.
However, six mothers who received TAK-003 in the exposure window and two neonates experienced serious AEs, but none were considered TAK-003 related.
These researchers identified a significant concern... administering live-attenuated vaccines during pregnancy is the potential for vertical transmission and a replicating vaccine virus posing a risk to the fetus.
The limitations of this post-hoc analysis include the small sample size of unintentional pregnancies occurring within the exposure window. Therefore, the quality and level of information about the pregnancies differed and depended on the extent of availability and level of antenatal care sought.
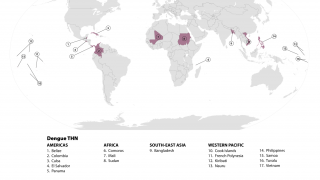
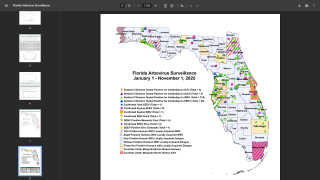
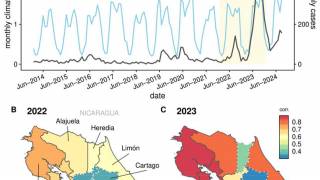
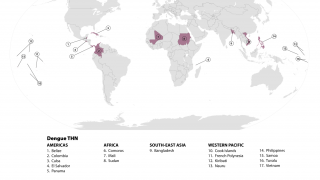
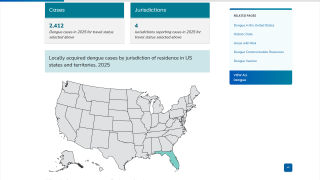
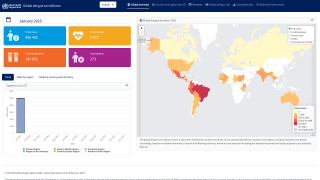
Currently, 46 countries and territories in the Americas report dengue outbreaks. About 1.2 million Dengue infections have already been reported in 2025.
As of March 19, 2025, the QDENGA vaccine remains unavailable in the United States.
Takeda Pharmaceuticals International AG, the producer of QDENGA, funded this study.
Our Trust Standards: Medical Advisory Committee