Gonorrhea is More Preventable and Treatable

Increasing antimicrobial resistance is just one barrier to controlling the spread of sexually transmitted infections (STIs) such as gonorrhea. However, new medications and better access to immunization products may reduce STIs in the United States.
A new antibiotic that was recently found to be as effective as the previous standard of care at treating urogenital gonorrhea has a new oral option for patients in the United States.
GSK plc today announced that the U.S. Food and Drug Administration (FDA) has approved a supplemental New Drug Application for gepotidacin as an oral option for adult and paediatric patients from 12 years of age weighing at least 45 kg who have limited or no alternative options for the treatment of uncomplicated urogenital gonorrhoea caused by susceptible strains of Neisseria gonorrhoeae.
This milestone follows the FDA approval of gepotidacin earlier in 2025 as an oral treatment for female adults and paediatric patients aged 12 years and older (weighing ≥40 kg) with uncomplicated urinary tract infection (uUTI).
Tony Wood, Chief Scientific Officer at GSK, stated in a press release on December 11, 2025: "We're proud to have delivered the first new class of antibiotics for gonorrhoea in over three decades and a new oral option for US patients."
"The ability of N. gonorrhoeae to develop resistance to currently available options, including standard of care, makes it important to expand the range of effective oral treatments".
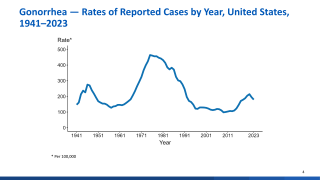
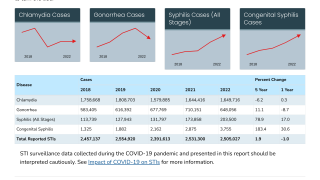
Gonorrhoea is a common, sexually transmitted infection caused by Neisseria gonorrhoeae, which the World Health Organization has recognised as a priority pathogen and the U.S. Centers for Disease Control and Prevention (CDC) has designated as an urgent public health threat. It is the second most commonly reported sexually transmitted infection in the USA.
While CDC states that there is currently no licensed vaccine the for the prevention of gonorrhoea infections, the United Kingdom has been offering certain people access to the off-label use of a FDA-approved vaccine, GSK's Bexsero® (MenB-4C).
In the UK, the NHS and local government in England and Wales have rolled out a world-first vaccine program to prevent gonorrhoea in May 2025. Real-world evidence has shown that Bexsero offers cross-protection against gonorrhea.
Our Trust Standards: Medical Advisory Committee