Will Dengue Passive Immunization Become Available in the USA
As travelers in the United States prepare for another year of record-setting Dengue fever outbreaks, a human monoclonal antibody (mAb) candidate from a Bethesda, MD-based company may soon obtain U.S. FDA approval.
As of February 12, 2025, the National Institutes of Health and AbViro LLC phase IIa clinical trial is exposing healthy volunteers in Maryland and Vermont to a weakened strain of the Dengue virus. The study will compare three AV-1 dose levels in adults challenged with DENV-3, the most infectious Dengue virus strain.
This study launched in early January 2025 and is expected to be completed this Fall.
If successful, the AV1 vaccine candidate could become the first dengue fever treatment approved by the FDA. Previously, the Dengvaxia® vaccine was approved but has since been withdrawn from the U.S. market.
Furthermore, the market-leading second-generation vaccine, Qdenga, is not approved in the U.S.
Currently, no approved Dengue vaccines or mAb are available in the U.S.
National Institute of Allergy and Infectious Diseases director Jeanne Marrazzo commented in a media release, "When caring for a patient who is critically ill with Dengue, healthcare providers have few options other than providing supportive care. We must find safe and effective therapeutics to provide much-needed relief to people suffering from Dengue."
While vaccines are designed to stimulate the body's immune system for many years, therapeutic mAbs provide rapid protection following injection.
Another mAb is being developed in India in a phase 3 study.
Dengushield, developed by Serum Institute of India Pvt. Ltd., is a highly potent inhibitor of all four types of dengue viruses. In a phase 1 study announced in February 2024, this mAb was found safe and well tolerated. It showed a dose-proportionate increase in pharmacokinetic exposure.
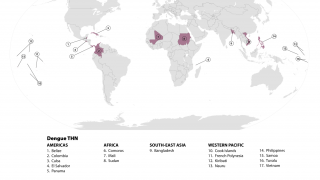
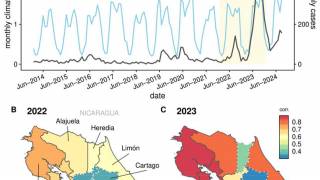
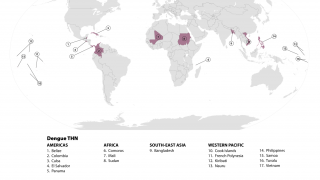
To alert international travelers to their Dengue risk, the CDC has issued Travel Health Advisories in 2025. Previously, the WHO classified Dengue as a grade 3 emergency, with an estimated 4 billion people at risk globally.
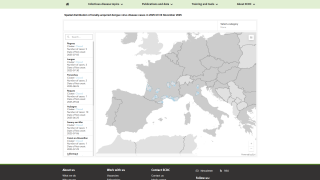
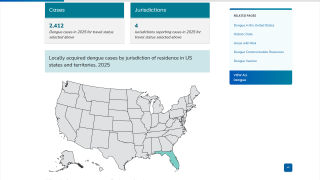
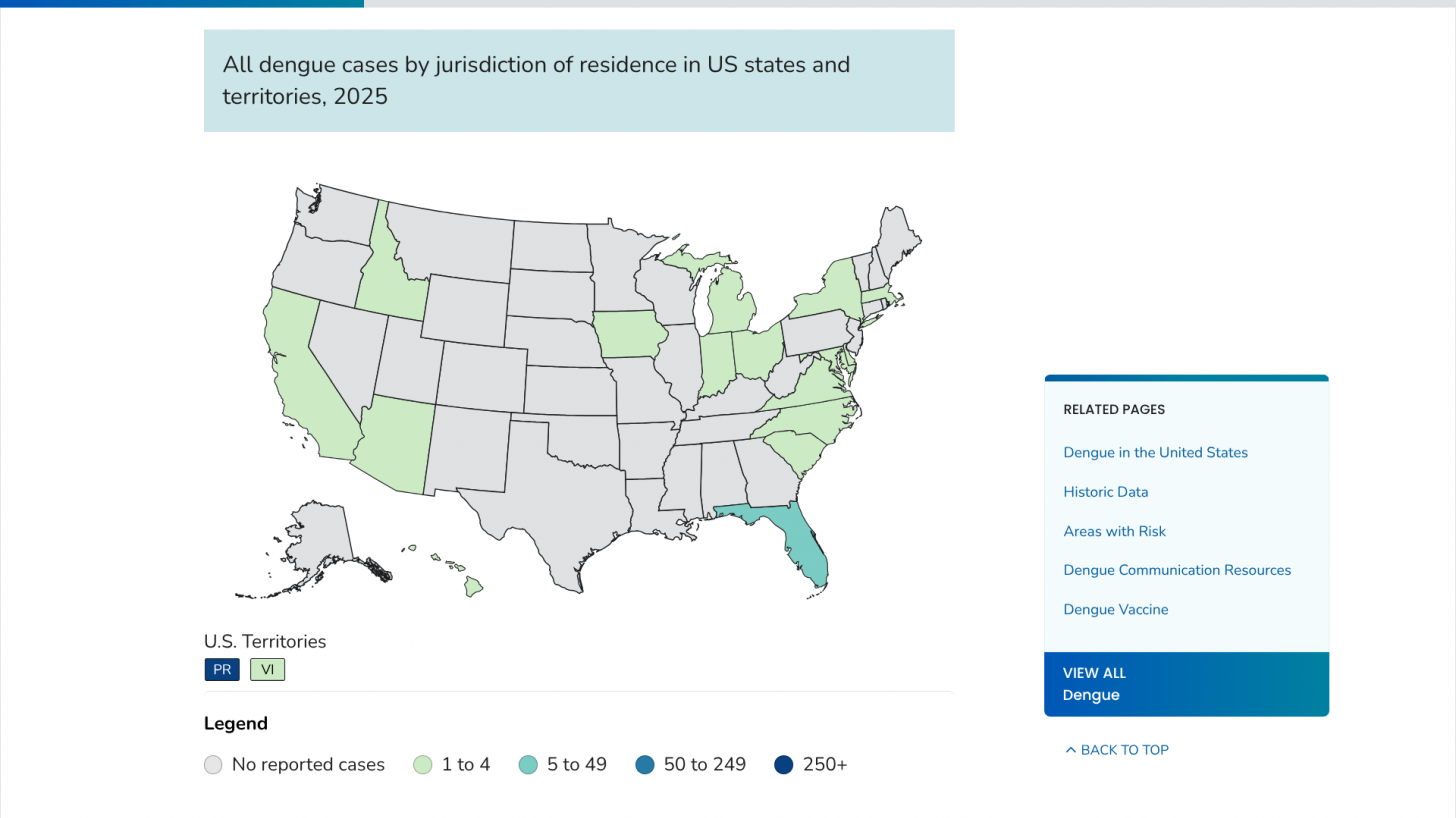
Within the U.S., Miami, Florida, and San Juan, Puerto Rico, are leaders in reporting locally acquired dengue cases.
Without an approved preventive vaccine or a mAb, the CDC suggests avoiding mosquito bites as the best defense against contracting Dengue in 2025.
Our Trust Standards: Medical Advisory Committee