Dengue Vaccinations Could Save $1.7 Billion Over Twenty Years

According to the World Health Organization (WHO) and numerous health agencies, Dengue is a viral infection and a leading cause of febrile illness among international travelers.
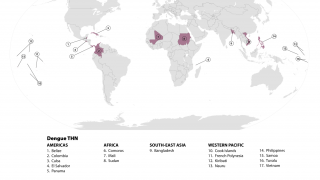
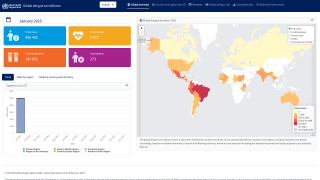
The WHO recently classified Dengue as a grade 3 emergency, with an estimated 4 billion people at risk globally.
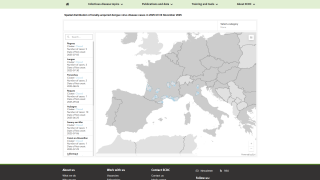
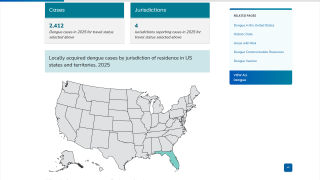
As of June 2025, the WHO Dengue Situation Update 723 indicates that over 6 million Dengue cases and 7,552 related fatalities have occurred in 2025, including cases in Florida.
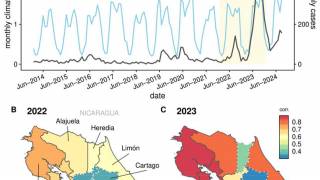
Following a record-setting number of Dengue Fever outbreaks in 2024, various countries have accelerated their plans to integrate a second-generation vaccine into vaccination programs, as there is no adequate treatment once infected.
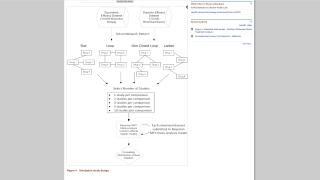
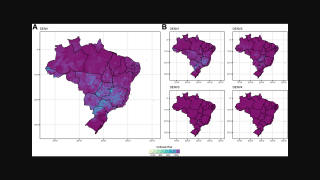
Based on the results of a Takeda-sponsored study, the public health impact and cost-effectiveness of launching an expansive vaccination program with the QDENGA® (TAK-003) two-dose vaccine would be significant.
On June 17, 2025, these researchers concluded that across a range of vaccination strategies explored in Thailand, QDENGA is estimated to prevent 41%–57% of disease cases and 47%–70% of hospitalizations over a 20-year period.
Among the explored vaccination strategies, routine vaccination of children, combined with 10 additional catch-up cohorts, would result in savings of $ 1.786 billion.
The study's findings are essential, as Dengue is the world's fastest-spreading vector-borne viral disease and one of the top 10 threats to human life. Dengue is primarily transmitted via the mosquitoes Aedes aegypti and Aedes albopictus, which are found in most countries.
As of June 20, 2025, QDENGA is the only licensed vaccine in about 30 countries that can be used for individuals both with and without a previous infection with any of the four Dengue viruses.
Currently, QDENGA is not available in the United States; however, the first-generation Dengvaxia® is being deployed in Puerto Rico.
Furthermore, Dengue vaccine candidates (Merck's V181, Butantan Institute Butantan-DV) are conducting late-stage clinical trials, which may lead to future approval by the U.S. Food and Drug Administration and potential availability in 2026.
An approved Dengue vaccine could reduce the number of travel-related cases (10) Texas has reported in 2025.
Note: This news article was updated on June 23, 2025.
Our Trust Standards: Medical Advisory Committee