Brazil's Dengue Vaccine Production to Reach 100 Million

The government of São Paulo, Brazil, through the State Department of Health, recently declared a state of public health emergency 'in view of dengue becoming epidemic.'
This agency says the 'cause of concern is the reappearance of the mosquito-transmitted dengue serotype 3 after seventeen years without circulation, as the Sao Pailo State population is not immune to this specific dengue strain.'
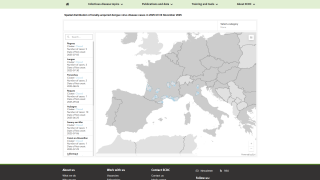
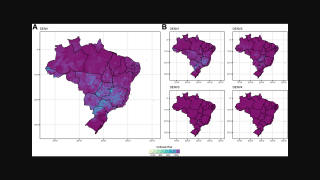
As of February 19, 2025, the emergency measure was adopted after the state of São Paulo reported 280 cases of dengue fever per 100,000 inhabitants in 2025, near the threshold for a state of emergency. State Secretary of Health Eleuses Paiva signed the state of emergency decree.
Data from the State Department of Health indicate more than 124,000 cases and 113 deaths throughout the state in 2025 alone, with 225 cities reporting more than 300 cases per 1,000 inhabitants, some of them in the northwest region.
Eleuses pointed out a series of measures to combat dengue fever in the state, including the vaccine developed by the Butantan Institute, which the National Health Surveillance Agency approves.
According to Secretary Paiva, the country will only be able to rid itself of the dengue epidemic that emerges yearly with large-scale vaccinations.
"We are providing the Butantan Institute with all the necessary infrastructure to ensure that we can produce the vaccine properly, as we are again experiencing a dengue epidemic. There is only one way to end dengue in our country, and that is to be able to put the vaccine in the arms of all Brazilians. In this sense, we have the full commitment of Butantan, the government, and the secretariat to focus on this," said Eleuses in a media release.
The director of the Butantan Institute, Esper Kallás, stated that the Institute made a Butantan-DV vaccine registration request on December 16, 2024. Butantan-DV is the only single-dose vaccine capable of neutralizing the four dengue serotypes.
"We ....intend to have the entire package sent back to Anvisa for consideration in the coming weeks," Kallás added.
"In 2025, we are working on the final stages of adjusting the processes and producing the initial batches, with the expectation of delivering 1 million doses, which would represent 1 million vaccinated people (in 2025)."
"In 2026, we would scale up and deliver another 60 million doses, and in 2027, another 40 million doses, to achieve the 100 million vaccination curve for the Brazilian population," explained Esper Kallás.
As of February 2025, the single-dose, tetravalent, live-attenuated Butantan-DV Dengue Vaccine is conducting phase 3 clinical trials. The vaccine results from a partnership between Butantan, the U.S. National Institutes of Health, and the American Type Culture Archive. Development of the tetravalent dengue vaccine began at Butantan Institute in 2010.
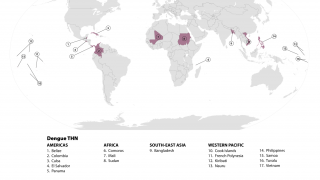
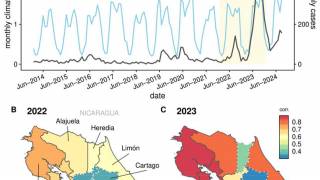
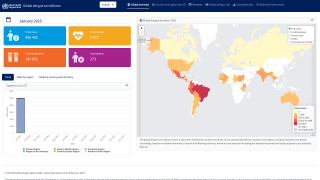
In addition to Brazil's needs, dengue vaccines are needed throughout the Region of the Americas, which set all-time case and fatality records in 2024.
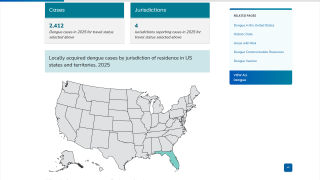
For example, Florida's southeast coast has reported locally-acquired dengue cases in the United States for the past few years.
On January 20, 2025, the World Health Organization classified dengue as a grade 3 emergency, with an estimated 4 billion people at risk globally.
When visiting a dengue outbreak area in 2025, the U.S. CDC suggests discussing disease prevention options with a travel vaccine expert. As of February 20, 2025, no dengue vaccines are offered in the U.S.
Our Trust Standards: Medical Advisory Committee