Dengue Vaccines
Dengue Vaccines 2026
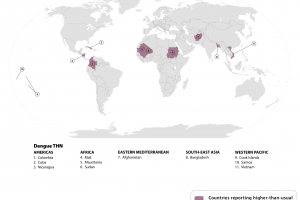
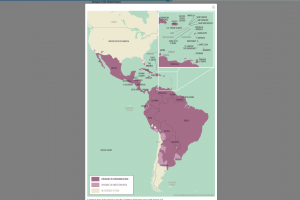
According to the World Health Organization (WHO), in 2026, Dengue is a vaccine-preventable disease, with approved vaccines available. As of January 6, 2026, the U.S. Food and Drug Administration (FDA) and the UK Medicines and Healthcare Products Regulatory Agency (MHRA), the Pan American Health Organization (PAHO), the European Medicines Agency (EMA), Australia's Technical Advisory Group on Immunisation, and Brazil's National Health Surveillance Agency (ANVISA) recommend dengue vaccination for specific individuals living in or visiting Dengue-risk areas, such as Puerto Rico and Brazil. The UK Health Security Agency published updated dengue vaccination guidance in the 'Green Book' (Chapter 15a) in October 2024.
Dengvaxia® is a live attenuated tetravalent chimeric vaccine approved by the U.S. FDA and various countries. Dengvaxia is no longer offered in the U.S., except for children in Puerto Rico, where Dengue fever has become endemic.
QDENGA® dengue vaccine is approved or authorized in various countries and does not require pre-admission testing. As of July 2025, the U.S. FDA has not approved this vaccine.
Butantan Institute's single-dose, Butantan-DV tetravalent dengue vaccine demonstrated 67-79% efficacy in preventing disease in Brazil, according to a Phase 3 clinical study. Butantan-DV is derived from a technology licensed from the U.S. National Institutes of Health in 2009. Under the collaboration agreement announced in December 2018, Merck and Instituto Butantan are sharing clinical study data for the vaccine.
Dengue Vaccine Candidates
As of 2026, clinical trials for the dengue vaccine candidate are recruiting new participants. A 2025 review highlights the challenges in developing a third-generation dengue vaccine. A study published in April 2025 found that the antibody avidity index is essential for characterizing protective DENV immune responses.
Merck's MOBILIZE-1 Phase 3 clinical trial is being initiated for V181, a live attenuated quadrivalent dengue vaccine candidate. As of June 12, 2025, the study will evaluate a single dose of V181 for the prevention of dengue disease caused by any of the four serotypes of the dengue virus, regardless of previous exposure.
Panacea Biotech, in collaboration with the Indian Council of Medical Research (ICMR), is developing the DengiAll dengue vaccine. The tetravalent dengue vaccine strain (TV003/TV005), initially created by the U.S. National Institutes of Health, has shown promising results in preclinical and clinical trials worldwide. Phase 1 and 2 clinical trials of the Indian vaccine formulation were completed in 2018-19, yielding promising results. With 3 years of follow-up, the single-dose tetravalent dengue vaccine, TV005, was well tolerated and immunogenic across all four serotypes in young children through adults, including individuals with no prior dengue exposure. Panacea Biotec has worked extensively on these strains to create a full-fledged vaccine formulation and holds a process patent. In August 2024, ICMR and Panacea announced the launch of a Phase 3 clinical trial in India.
Serum Institute of India's tetravalent dengue vaccine live candidate, Dengusiil, is conducting phase 2 clinical research in 2024. A previous Phase 1 study concluded that a single dose of Dengusiil was safe and well-tolerated in adults, and highly immunogenic, with trivalent or tetravalent seroconversion and seropositivity in most participants (69%).
National Institute of Allergy and Infectious Diseases - TetraVax-DV T005 (rDEN3Δ30/31-7164) is a live attenuated tetravalent vaccine. A Phase 2 clinical trial revealed that, with three years of follow-up, a single dose of TV005 was well-tolerated and immunogenic for all four serotypes in young children and adults, including individuals with no prior dengue exposure. Results of a phase 2 study of TV005 in Bangladesh, published in 2024, reported the waning of antibody titers in children aged 1–4 years who were seronegative at the time of vaccination. Only 22–28% of children in this age group remained seropositive for DENV-1, DENV-3, and DENV-4 after three years of follow-up, compared with 69% seropositivity for DENV-2.
TetraVax-DV-TV003 (V180) is a live-attenuated, recombinant, tetravalent investigational dengue vaccine, currently undergoing a Phase 3 clinical trial in Brazil. Dr. Stephen Whitehead's laboratory developed the vaccine. Merck recently completed a phase 1 study (V180-001).
The DV1-DV4 vaccine candidate is transitioning into a human clinical study. This new vaccine construct, which comprises Nature's gene-chip peptides bound to a quantum-cluster gold nanoparticle delivery system, demonstrated an excellent safety profile in a repeat-dose Good Laboratory Practice (GLP)-grade toxicology study using a standard industry model.
Àvida Biotech's novel oral vaccine candidate for Dengue does not require cold transport or storage and has completed proof of concept in a mouse model. Additionally, the University of Buffalo's Center for Integrated Global Biomedical Sciences will provide expertise in preclinical drug development.
K.M. Biologics' KD-382 vaccine is a live-attenuated tetravalent dengue candidate in Phase 1 clinical trials. A single dose is expected to be effective against all four serotypes. Additionally, this live attenuated virus vaccine is expected to induce neutralizing antibodies and cellular immunity, similar to that caused by natural infection.
Emergex PepGNP-Dengue DengueTcP™, Its Novel T Cell-Priming Immune Set-Point Candidate, uses 100% synthetic vaccines to 'prime' naive CD8+ T-Cells to generate virus-specific CTL (CD8+ Cytotoxic T Lymphocyte cells) to kill infected cells before productive viral infection, thus preventing viral replication and disease in the vaccinated person. naNO-DENGUE: A Phase-I study of a nanoparticle-based peptide vaccine against Dengue virus.
CodaVax-DENV is a next-generation, tetravalent, live-attenuated dengue vaccine candidate under development by Codagenix Inc. The Company's vaccine design platform has enabled the precise and rational attenuation of contemporary serotypes of all four dengue virus strains through selective codon deoptimization. With this approach, Codagenix can rationally balance all four virus serotypes to produce a safe and highly immunogenic vaccine. On October 24, 2023, Codagenic announced that the U.S. Department of Defense awarded the Company $5.88 million to advance the development of its CodaVax-DENV program. The funding supports good manufacturing practices of drug substances and tetravalent drug products for a Phase 1 study and a first-in-human Phase 1 safety and immunogenicity trial. This award complements a $4.4 million Department of Defense (DoD) award granted in 2022.
Indian Immunologicals Limited (IIL) anticipates launching its dengue fever vaccine commercially by 2026. IIL's Managing Director, K. Anand Kumar, stated on August 20, 2024, that the vaccine's early-stage trials involving approximately 90 individuals aged 18-50 did not demonstrate any adverse effects. The U.S. National Institute of Health provided IIL with the dengue virus required to develop the vaccine. ILI is a subsidiary of the National Dairy Development Board.
Dengue Monoclonal Antibody Therapy
Dengushield (VIS513) is a humanized monoclonal antibody (mAb), not a vaccine, that provides passive immunization and is evaluated in phase 2 clinical trials for the treatment of Dengue. VIS513 is a highly potent inhibitor of all four dengue virus serotypes, both in vitro and in preclinical animal models. VIS513 was licensed to the Serum Institute of India Pvt. Ltd (SIIPL) for development and commercialization may cost 5,000 to 10,000 rupees per dose. SIIPL funded a Phase 1 study announced in February 2024, and the VIS513 mAb was found to be safe and well-tolerated, with a dose-proportionate increase in pharmacokinetic exposure. A phase 3 study is ongoing.
AbViro LLC (Janssen, LP) AV-1 dengue mAb candidate (JNJ-64281802) is conducting a Phase 2, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study to Compare the Safety and Efficacy of Three Dose Levels in Healthy Adults Challenged With a Controlled Human Infection Strain of DENV-3. Before or after AV-1 dosing, each volunteer will receive an attenuated (weakened) dengue virus injection. If AV-1 shows promising results in this clinical trial, researchers may pursue further clinical evaluations of its safety and efficacy against the dengue virus. In earlier studies using this challenge virus, most volunteers developed a rash, and some had other mild dengue symptoms, such as joint and muscle pain or headache. None of the volunteers developed dengue fever or severe Dengue.