Brazil to Launch Virus-like Particle Chikungunya Vaccine in 2027
Bavarian Nordic A/S today announced an agreement with Eurofarma, granting the Brazilian pharmaceutical company exclusive rights to sell and distribute Bavarian Nordic's chikungunya vaccine, CHIKV VLP (VIMKUNYA®), in Brazil.
Additionally, under the terms of the agreement announced on January 23, 2026, Eurofarma will have the right of first refusal for any future opportunities to register and commercialize the virus-like particle (VLP) vaccine in the rest of Latin America.
According to the press release, pending discussions with the Brazilian Health Regulatory Agency (Anvisa), a regulatory submission is anticipated in the first half of 2026, which could support a potential launch of the vaccine in Brazil in the latter half of 2027.
Chikungunya is a mosquito-borne viral disease caused by the chikungunya virus. Over the past two decades, chikungunya has been reported in over 110 countries. Since 2013, the virus has been detected in the Americas Region.
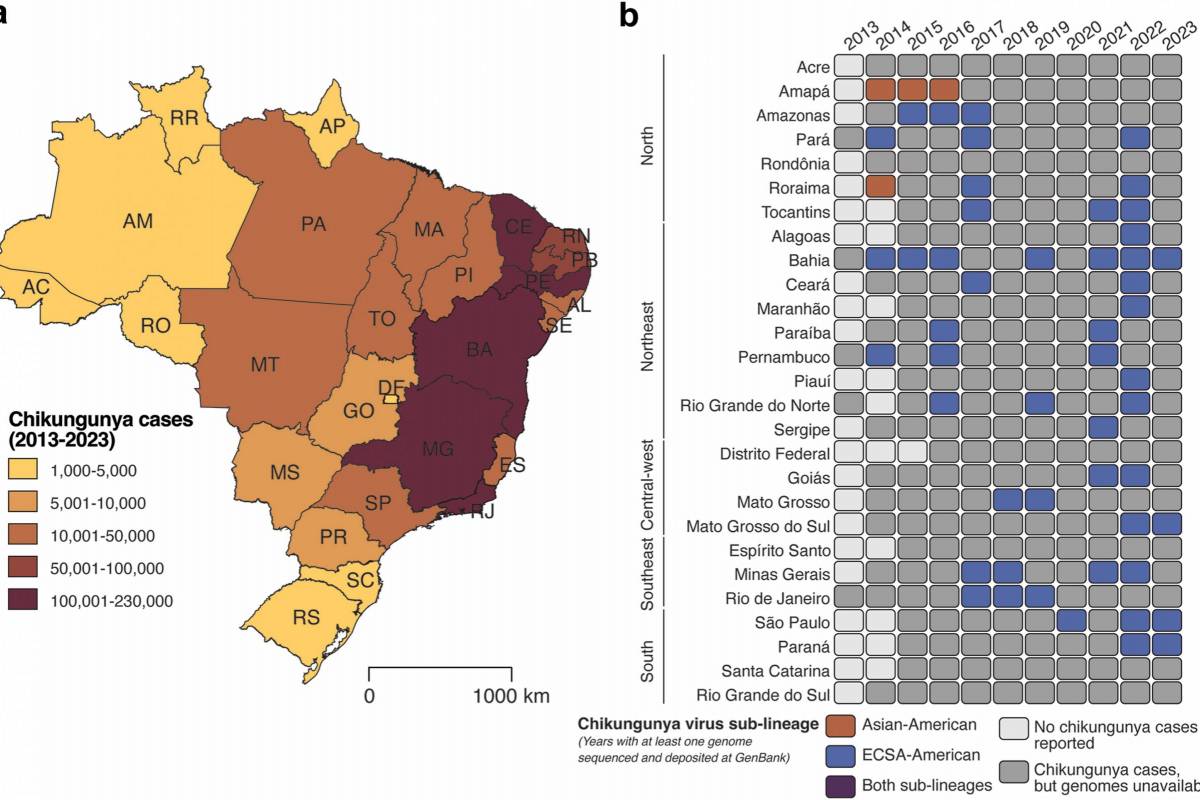
Brazil remains one of the most heavily affected countries, having reported a significant share of global cases and hospitalizations in recent years.
In 2025, Brazil reported over 250,000 chikungunya cases and 120 related fatalities.
Paul Chaplin, President & CEO of Bavarian Nordic, commented, "Brazil continues to bear a significant burden from chikungunya, accounting for a substantial portion of global reported cases and related deaths."
In the United States, both travel-related and locally acquired chikungunya have been confirmed in Florida in early 2026. During 2025, numerious cases were related to travelers from Cuba.
CHIKV VLP is a single-dose, prefilled, adjuvanted VLP recombinant protein vaccine designed for the active immunization and prevention of disease caused by the chikungunya virus.
The vaccine contains no viral genetic material, making it non-infectious and unable to cause disease, thereby providing a favorable safety profile suitable for a broad range of individuals.
VIMKUNYA® was approved by the U.S. Food and Drug Administration in February 2025, by the European Commission in February 2025, and by the United Kingdom in May 2025, initially for use in travelers and at-risk populations aged 12 years and older.
This is the second chikungunya vaccine targeting Brazil.
In April 2025, Anvisa granted marketing authorization to its single-dose vaccine IXCHIQ®.
As of January 24, 2026, VIMKUNYA® is commercailly offered at travel clinics throughout the U.S.
For more information, visit www.bavarian-nordic.com.
Our Trust Standards: Medical Advisory Committee