Philadelphia health officials are raising concerns about a noticeable increase in chickenpox (varicella) cases among unvaccinated children in the city.
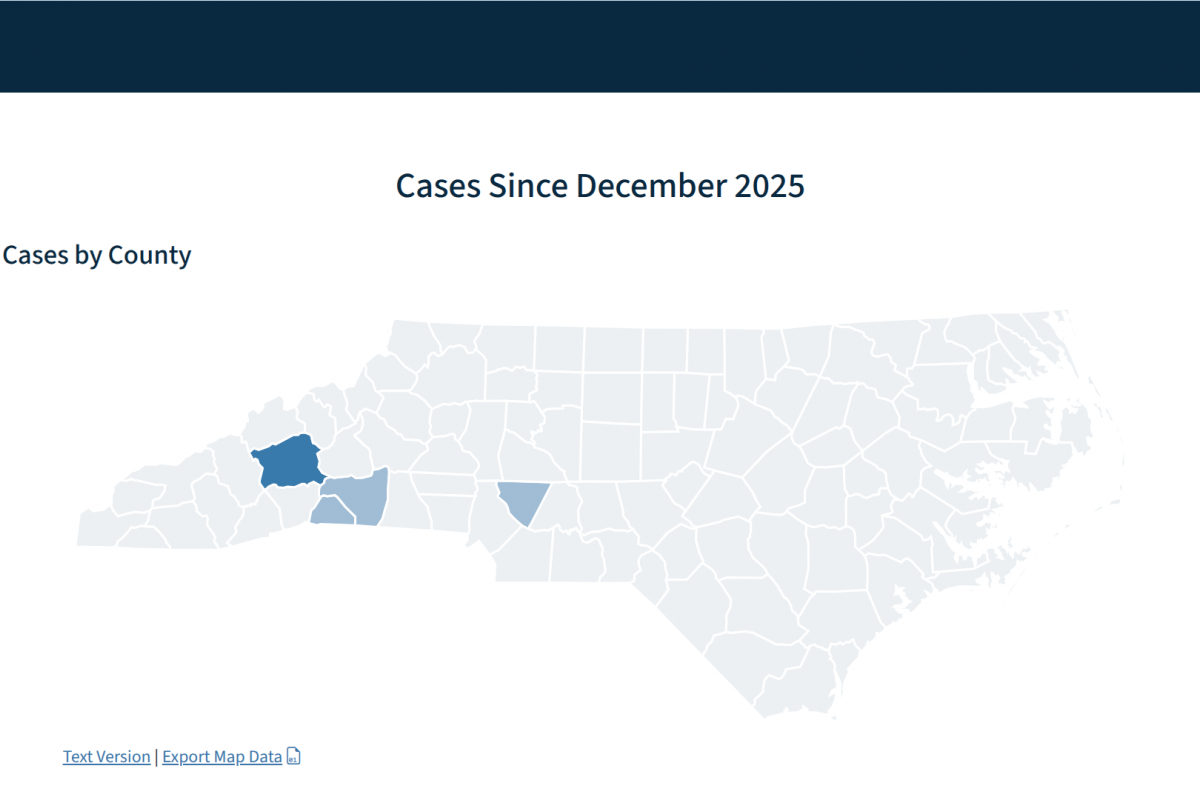
In early 2026, the Philadelphia Department of Public Health (PDPH) reported two separate outbreaks at two schools in eastern Pennsylvania.
On January 5, 2026, the PDPH issued a health advisory to clinicians and the public. The advisory emphasized the importance of recognizing, managing, controlling, and preventing varicella.
In November 2025, PDHP issued a similar health alert about chickenpox cases in schools.
This situation underscores ongoing worries about vaccine-preventable diseases in schools. Currently, the student population in Philadelphia exceeds 1.6 million from kindergarten through 12th grade.
Gayle Mendoza, a spokesperson for the PDPH, told the Inquirer on January 27, 2026, that she had no details on how many cases were reported among vaccinated versus unvaccinated children. It's also unclear which factors have contributed to the rise in chickenpox cases.
Although the current outbreaks are limited in scope, officials stress the necessity of maintaining high vaccination rates to prevent a wider spread of this once-common childhood illness.
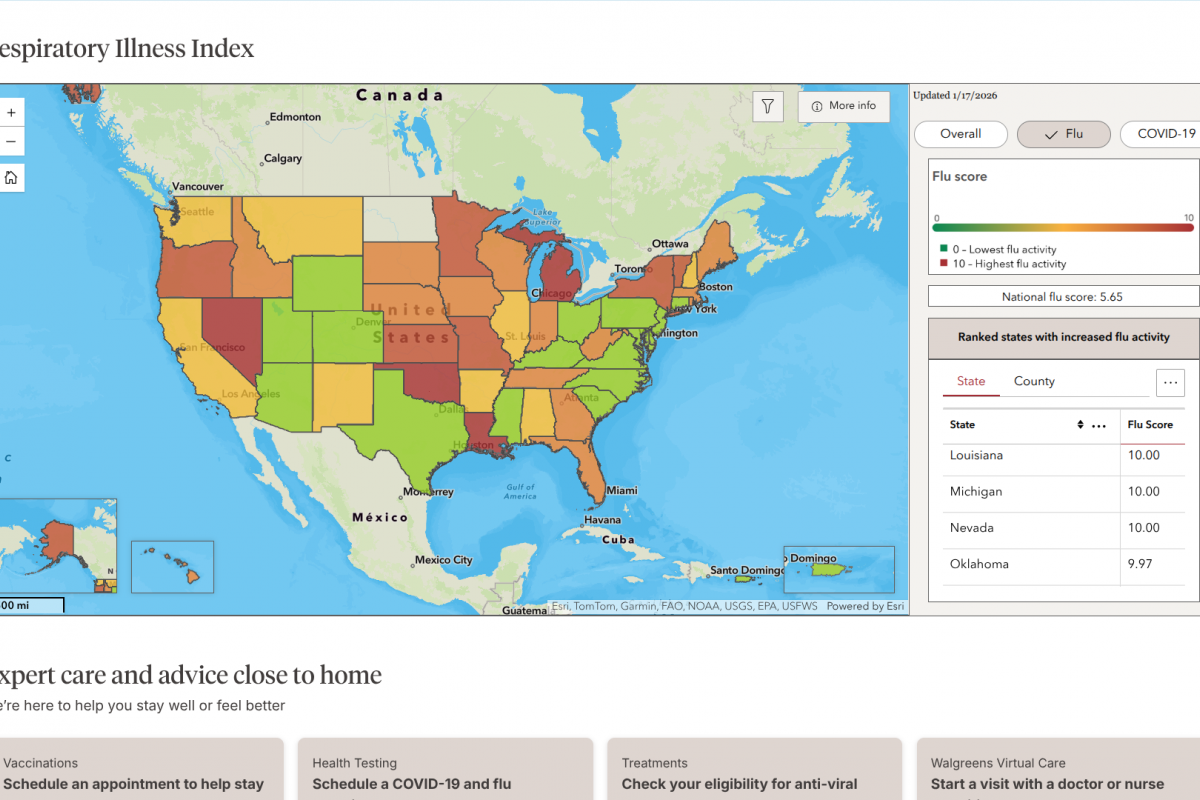
During 2025, the U.S. CDC data indicates fewer than 150,000 cases reported annually in recent years. Hospitalizations were under 1,400, and deaths were around 30 or fewer.
Chickenpox can pose serious risks to vulnerable populations, including pregnant individuals, newborns, and those with weakened immune systems.