MERS Vaccines
Middle East Respiratory Syndrome (MERS) Vaccine Candidates 2026
The U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), the World Health Organization (WHO), and the Kingdom of Saudi Arabia have not approved a Middle East Respiratory Syndrome Coronavirus (MERS-CoV) vaccine candidate as of January 2026. The WHO says several vaccine candidates are being tested in human clinical trials in 2025. Efforts to develop an effective and safe human MERS-CoV vaccine have advanced, with a few vaccine candidates entering human studies; these vaccines are based on DNA plasmid vectors and modified vaccinia Ankara.
On April 10, 2023, the U.S. government announced Project-NextGen, which aims to empower companies to accelerate the development of vaccines and therapies for human coronaviruses, such as MERS.
MERS-COV Vaccine Candidates
Oxford University's Pandemic Sciences Institute and Barinthus Biotherapeutics Inc. developed the ChAdOx1 MERS vaccine and announced on September 15, 2023, that 84 people aged 50 to 70 would participate in a phase 1 study in Liverpool. This study builds upon two previous Phase I clinical trials conducted in the UK in 2018 and Saudi Arabia in 2019. VTP-500 (ChAdOx1) MERS-CoV is a vaccine candidate from the University of Oxford. It consists of the replication-deficient simian adenovirus vector ChAdOx1 ME, expressing the RS Spike protein antigen. The VTP-500 vaccine is administered as a single dose in a homologous prime-boost regimen. C regimenEPI is funding up to $34.8 million to develop and stockpile VTP-500 vaccines. Due to VTP-500's potential to significantly address the unmet need for MERS, the EMA has confirmed support for the program through the PRIME designation.
The Universitätsklinikum Hamburg-Eppendorf MVA MERS-S (Modified Vaccinia virus Ankara) vaccine candidate contains the full-length spike (S) gene of SARS-CoV-2. Vaccination with MVA-MERS-CoV had a favorable safety profile, with no severe adverse events reported. A Phase 1b study, concluded in October 2024, found that MVA-MERS-S was safe and immunogenic in individuals with previous and concurrent SARS-CoV-2 exposure.
BVRS-GamVac-Combi is conducting Phase 1/2 clinical studies, sponsored by the Gamaleya Research Institute of Epidemiology and Microbiology, which is part of the Russian Federation's Ministry of Health.
The inactivated rabies-vectored RS-CoV-2 S1 vaccine, CORAVAX, is adjuvanted with MPLA-AddaVax, a TLR4 agonist, and induces high levels of neutralizing antibodies, thereby generating a strong Th1-biased immune response. The Avaccc 101 vaccine candidate is designed to provide broad protection against SARS-CoV-1, SARS-CoV-2, and MERS-CoV.
Novavax's MERS investigational vaccine was paused at the preclinical stage.
Ralph Baric's lab at the University of North Carolina at Chapel Hill, in collaboration with the U.S. NIAID, negotiated an agreement to develop an MERS mRNA vaccine candidate.
CEPI provided $2.6 million in March 2025 to advance Uvax BioBio's RS vaccine candidate into preclinical trials.
MERS Testing
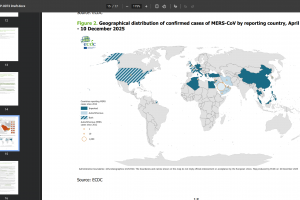
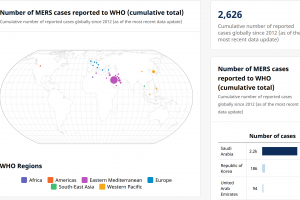
The U.S. Centers for Disease Control and Prevention (CDC) describes MERS-CoV as a viral respiratory infection. The zoonotic source of this virus remains unknown. Since April 2012, when a patient with pneumonia died in a Jeddah hospital in Saudi Arabia, health authorities from 27 countries in six World Health Organization regions have reported 2,627 cases of MERS, including 946 deaths.
The U.S. CDC recommends MERS-CoV testing for persons within the United States who meet the MERS-CoV person-under-investigation criteria. In the United States, MERS-CoV testing declined from 2017 to 2023, and the clinical and epidemiologic criteria to guide U.S. testing were updated in 2024.
A study published in The Lancet in July 2024 highlighted the potential threat posed by RS-CoV, a member of the genus Merbecovirus, to global health. MERS-CoV circulates in dromedary camels in the Arabian Peninsula and occasionally causes human spillover infections. The emergence of MERS-CoV in camels and humans was preceded by a critical recombination event in which the ancestral receptor-binding module was replaced with a different merbecovirus lineage, thereby altering receptor usage. A key concern is the virus's ability to utilize diverse cell entry receptors, including ACE2.
The U.S. CDC's Emerging Infectious Diseases published a study (Volume 30, Number 3) in March 2024 that identified more than three clusters among animals from different areas of Nairobi, Kenya, and a 15% infection rate among slaughterhouse workers.