Malaria Vaccines
Malaria Vaccines 2025
The World Health Organization (WHO) recommends the use of malaria vaccines to prevent P. falciparum malaria in children living in malaria-endemic areas. As of November 2025, the WHO and the European Medicines Agency (EMA) recommend Mosquirix™ and R21/Matrix-M™ vaccines for travelers visiting malaria-endemic countries. These malaria vaccines were added to the WHO's list of prequalified vaccines in 2024.
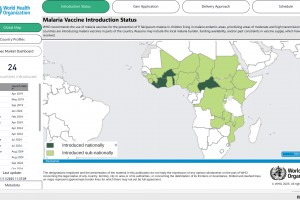
As of 2025, 24 countries have introduced malaria vaccines through routine immunization targeting children. Fourteen of these countries introduced the vaccines in 2024, including Cameroon, Burkina Faso, Sierra Leone, Benin, Liberia, Côte d'Ivoire, South Sudan, Mozambique, Central African Republic, Niger, Chad, Democratic Republic of Congo, Sudan, and Nigeria. Ghana, Kenya, and Malawi introduced the first malaria vaccine in 2021 and have since scaled up routine provision of the vaccine through Gavi support. In 2025, 6 to 8 countries, including Uganda, Ethiopia, Guinea, Mali, and Burundi, plan to offer malaria vaccinations.
The WHO's World Malaria Report 2024 estimates that the annual global demand for malaria vaccines will be 40–60 million doses by 2026 and 80–100 million doses annually by 2030. In November 2024, the WHO published a revised version of the Consolidated Guidelines for Malaria, which included an updated vaccine recommendation. People can be tested for malaria infection at commercial labs in the U.S.
Malaria Vaccines Approved
Mosquirix™ RTS, S/AS01 malaria vaccine. Mosquirix is a recombinant vaccine of the P. falciparum circumsporozoite protein from the pre-erythrocytic stage.
R21/Matrix-M™ was co-developed by the Institute of India, was designed in 2011, and co-produced by scientists at the University of Oxford, Novavax AB, and Novavax Inc.
As of 2025, the U.S. Food and Drug Administration had not approved a malaria vaccine.
Malaria Vaccine Candidates 2025
ProC6C-AlOH/Matrix-M is a multistage malaria vaccine, ProC6C, based on distinct Plasmodium falciparum epitopes from the sporozoite stage (P falciparum circumsporozoite protein [PfCSP]) and the transmission stages (Pfs230 and Pfs48/45), adsorbed to aluminium hydroxide (AlOH) and adjuvanted with Matrix-M adjuvant (ProC6C-AlOH/MM), has previously shown safety and immunogenicity in phase 1 studies. The vaccine achieved an efficacy of 54% (95% CI 9–77, p=0·029) at 12 weeks after the final dose, and 76% (95% CI 36–91, p=0·0022) in a time-to-event analysis in a phase 2 study, indicating a promising level of protection.
RH5.1/Matrix-M malaria vaccine, developed at the University of Oxford, targets the blood-stage malaria, unlike previously approved malaria vaccines that target the pre-erythrocytic stage. The results of the Phase 2b trial by Natama and colleagues indicated that RH5.1/Matrix-M had a vaccine efficacy of 55% (95% CI, 2-75%; p = 0.00711) when administered at 0, 1, and 5-month intervals. On December 10, 2024, and February 2025, researchers wrote, 'RH5.1/Matrix-M appears safe and highly immunogenic in African children and shows promising efficacy against clinical malaria when given in a delayed third-dose regimen.'
Sanaria Inc.'s non-replicating whole-parasite sporozoite (SPZ) vaccine candidate is made from a live-attenuated form of the malaria parasite Plasmodium falciparum sporozoite. Clinical studies have demonstrated approximately 0% protection in a challenging clinical trial. On August 14, 2024, The Lancet published clinical trial results showing that PfSPZ vaccination without presumptive antimalarial treatment before the first vaccine dose was ineffective in the preconception trial. A related editorial suggested that SPZ vaccination might avert malaria-related early pregnancy losses and reduce malaria-related anemia risk during the periconception period, with reductions of 65 to 86%.
The Lancet Infectious Diseases, published in July 2023, reported results from a phase 1 study evaluating the effectiveness of the Gamete vaccine Pfs230D1-EPA/Alhydrogel and the zygote vaccine Pfs25-EPA/Alhydrogel. Pfs230D1, but not the Pfs25 vaccine, induces durable serum functional activity in Malian adults.
Versatope Therapeutics Inc. is developing a bi-specific malaria vaccine targeting the initial stages of infection and transmission.
In a phase 1 study, the ProC6C-AlOH/Matrix-M vaccine candidate elicited the highest levels of functional antibodies, meriting further investigation.
BNT165e mRNA Malaria Vaccine candidate—BioNTech is developing the first malaria vaccine based on mRNA technology to eradicate mosquito-borne illness. The phase 1 clinical trial will evaluate the safety, tolerability, and exploratory immunogenicity of the 3-dose vaccine candidate. In March 2025, the U.S. FDA placed a clinical hold on the phase 1/2a study. BioNTech's Malaria project was first announced in July 2021.
Ocean Biomedical has been awarded a new patent for a parasite target called PfCDPK-5. This target could prevent the parasite at multiple stages of the malaria life cycle in a multivalent mRNA-based malaria vaccine. In addition, recent studies in Nature identified PfGARP as a target of human antibodies that kill up to 100% of parasites in vitro.
AgTRIO mRNA-lipid nanoparticle was assessed for its potential as a malaria vaccine.
repRNA Malaria Vaccine Technology
In January 2025, MalarVx, Inc. licensed HDT Bio's proprietary self-amplifying replicon RNA (repRNA) and lipid nanoparticle (LION™) technologies for use in malaria vaccines. MalarVx has also demonstrated the potential of LION-based vaccines to prevent infections caused by Plasmodium parasites, the pathogens responsible for malaria in humans and other animals.
Malaria Monoclonal Antibody Passive Immunization
The Lancet published results from a Phase 1 clinical trial on September 23, 2025, which concluded that MAM01 was well tolerated and met safety targets, and demonstrated clinical proof of principle by eliciting protection in malaria-naive adults using the controlled human malaria infection model.
The New England Journal of Medicine published the results of a study on April 26, 2024. The study demonstrated that a single subcutaneous injection of the NIAID's experimental L9LS (VRC-MALMAB0114-00-AB) malaria monoclonal antibody provided up to 77% protection against P. falciparum infection and clinical malaria over six months. According to the study authors, "the data from our trial support the administration of a single dose of L9LS to school-aged children before the malaria season."
The Phase 2 NIAID-USTTB evaluated the safety and efficacy of a single intravenous infusion of a monoclonal antibody called MALMAB0100-00-AB (CIS43LS). The antibody was up to 88.2% effective at preventing infection over 24 weeks, demonstrating for the first time that a monoclonal antibody can prevent malaria infection in an endemic region. This antibody has been previously shown to neutralize the sporozoites of P. falciparum in the skin and blood before they can infect liver cells.
Malaria Treatments
The 2024 World Malaria Report revealed a concerning trend: the global malaria incidence rate was 58.6 cases per 1000 people at risk in 2023. Various antimalarial treatments were approved by the U.S. CDC in 2025.