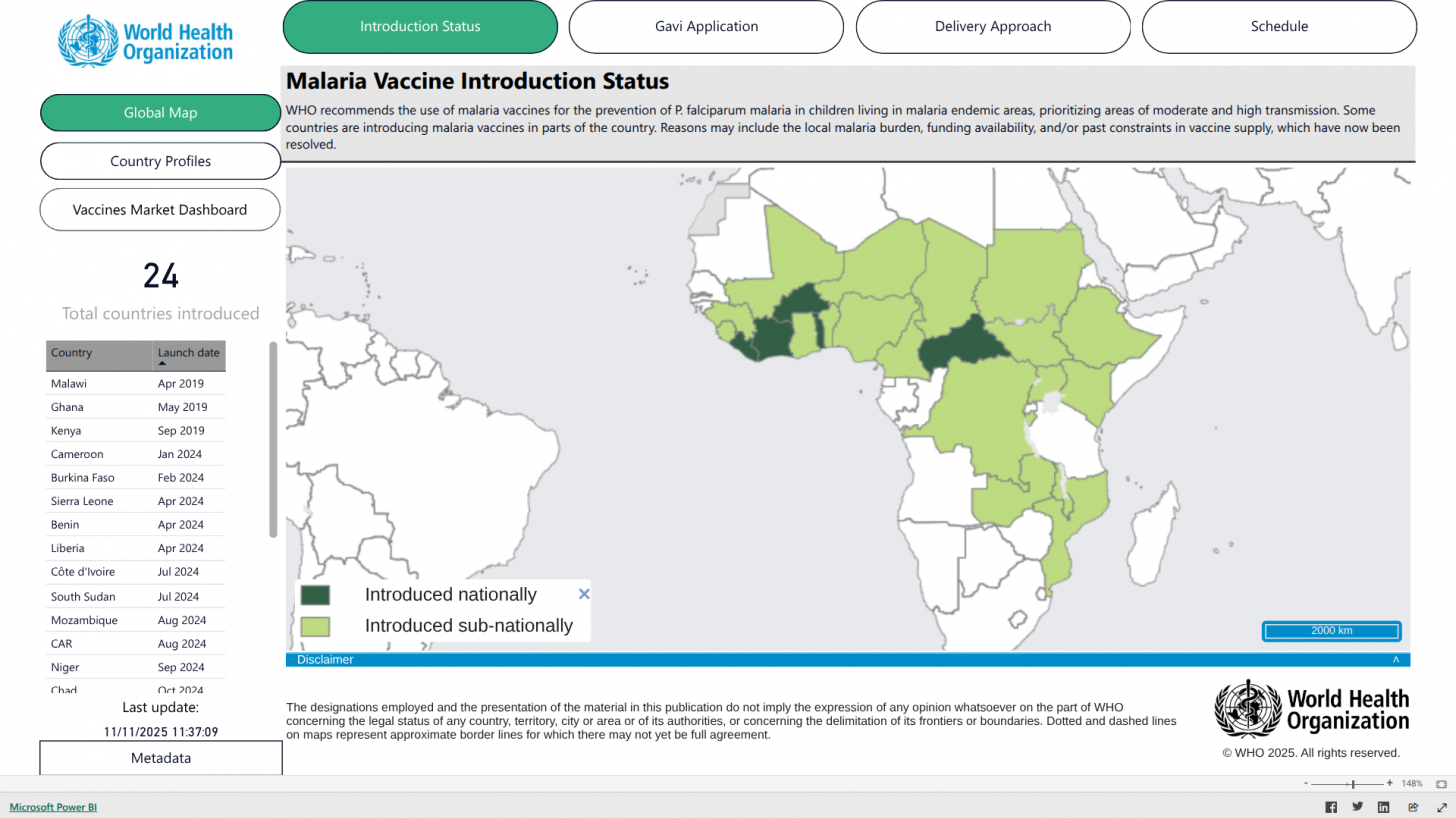
Effective Malaria Vaccines Offered in 24 Countries
A recent analysis of a phase 4 clinical trial conducted in Africa has shown that an approved malaria vaccine significantly decreases the impact of this mosquito-borne disease in children.
Malaria sickened an estimated 263 million people and caused 597,000 fatalities primarily in Africa in 2023.
The researchers reported their findings from this GSK-funded post-marketing study in The Lancet Global Health on November 6, 2025. After 1 year of follow-up following the third dose of Mosquirix™ (RTS,S/AS01E), there was a significant reduction in malaria incidence.
The researchers stated that these findings support the continued use of Mosquirix™ vaccination in children as an effective public health measure to reduce malaria-related illness and mortality in regions where the disease is endemic. They also emphasized the vaccine's importance for future malaria control strategies.
Mosquirix™ became the first malaria vaccine in 2021 to be recommended by the World Health Organization (WHO) for the prevention of Plasmodium falciparum malaria in areas of moderate-to-high transmission. The vaccine is given on a four-dose schedule.
In 2023, the WHO recommended a second four-dose malaria vaccine, R21/Matrix-M, for use in children. This vaccine, produced by the Serum Institute of India, includes Novavax's adjuvant and was developed by scientists at the University of Oxford.
The WHO estimates the two vaccines could prevent up to half a million child deaths by 2035 if scaled up in moderate- and high-transmission areas.
Currently, these malaria vaccines are unavailable in the United States.
If approved, a vaccine would reduce the approximately 2,000 malaria cases a year that are reported in the USA, and on average, there were nearly seven deaths per year for the period 2007 – 2022.
In Florida, 41 travel-related malaria cases have been confirmed in 2025, 15 related to travel to Nigeria.
Our Trust Standards: Medical Advisory Committee