Infants Can Now Receive Targeted Malaria Treatment
Every year, approximately 30 million infants are born in regions of Africa at risk for malaria infection. Without specific treatment for these vulnerable infants, malaria can quickly lead to complications and become fatal.
Until now, treatments have only been approved for infants weighing more than 4.5 kilograms, resulting in a significant treatment gap.
On July 8, 2025, Novartis announced that Swissmedic had approved Coartem® Baby (Riamet® Baby) as the first malaria medication specifically for newborns and young infants.
Previously, malaria-infected infants were treated with medications meant for older children, which raised the risk of overdose and toxicity.
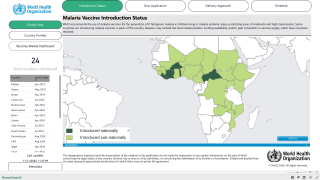
Additionally, the two approved malaria vaccines are not authorized for the youngest babies.
“For more than three decades, we have stayed the course in the fight against malaria, working relentlessly to deliver scientific breakthroughs where they are needed most,” said Vas Narasimhan, CEO of Novartis, in a press release on July 8, 2025.
“Together with our partners, we are proud to have gone further to develop the first clinically proven malaria treatment for newborns and young babies, ensuring even the smallest and most vulnerable can finally receive the care they deserve.”
Novartis plans to introduce the infant-friendly treatment on a primarily not-for-profit basis to increase access in areas where malaria is endemic.
Eight African countries also participated in the assessment and are now expected to issue rapid approvals under the Swiss agency’s Marketing Authorization for Global Health Products procedure.
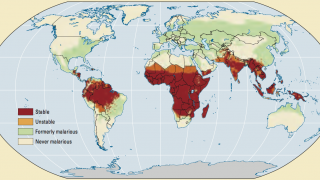
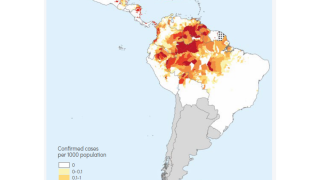
According to the Africa CDC, Malaria is a life-threatening disease caused by a parasite spread to humans by mosquitoes. According to the most recent data, children under 5 years old accounted for over 70% of malaria deaths in the African region.
The Swissmedic approval is based on the Phase II/III CALINA study. Coartem® Baby is indicated for the treatment of infants and neonates weighing between 2 and 5 kilograms with acute, uncomplicated infections due to Plasmodium falciparum or mixed infections including P. falciparum.
Currently, the availability of Coartem® Baby in the United States is undisclosed.
Our Trust Standards: Medical Advisory Committee