2026 Tuberculosis Outbreaks: Ending With Innovative Vaccines

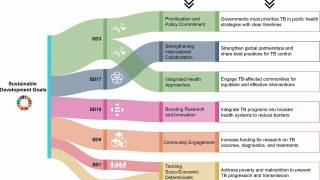
The World Health Organization (WHO) Executive Board's 158th session recently made a significant advancement in the global fight against tuberculosis (TB) by approving the development of a post-2030 End TB Strategy.
This decision, made in January 2026 during the Board's meeting, indicates a long-term commitment to sustain and accelerate progress beyond the existing 2030 targets of the current End TB Strategy.
This development comes at a crucial time, as renewed parliamentary mobilization has increased in anticipation of World TB Day on March 24, 2026. The 2026 theme, "Yes! We can end TB!", serves as a strong call to action and a message of hope, emphasizing the need for decisive leadership by countries, increased investment, and the prompt adoption of WHO recommendations to combat the rising TB epidemic.
According to the WHO Global Tuberculosis Report 2025, released in November 2025 and covering data from 2024, TB remains the leading cause of death from a single infectious agent worldwide.
In 2024, an estimated 10.7 million people became ill with TB, resulting in 1.23 million deaths. Notably, the WHO continues to highlight detection gaps, with approximately 2.4 million people either undiagnosed or unreported, especially in high-burden settings.
A February 2026 study published in Nature Medicine has raised concerns about the potential for overdiagnosis, suggesting that many reported TB cases globally may be inaccurate due to diagnostic limitations. This underscores the urgent need for improved testing tools.
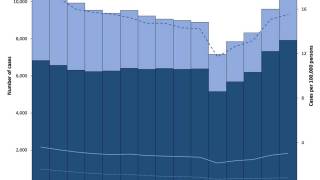
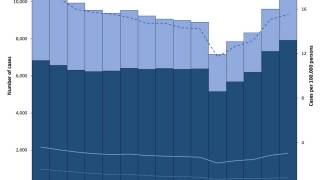
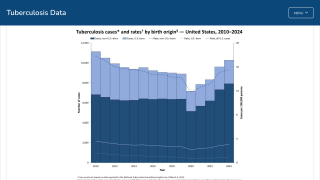
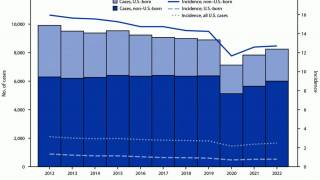
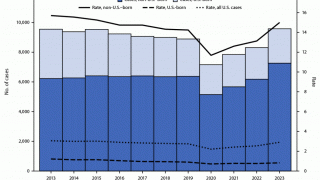
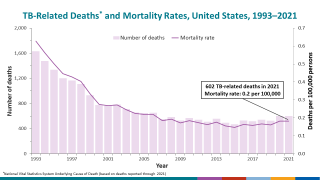
In the United States, TB rates have been rising for several years, but they remain among the lowest in the world.
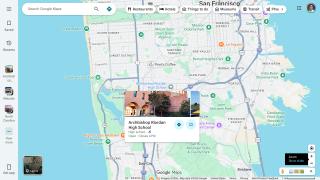
Final data from the U.S. CDC for 2024, released in December 2025, show 10,388 cases, reflecting a 7.9% increase in cases and a 6.9% rise in rates from 2023, which recorded 9,622 cases. While provisional data for 2025 is not yet available, ongoing localized outbreaks—such as one reported in January 2026 at Archbishop Riordan High School in San Francisco.
This outbreak highlights persistent challenges in the U.S., such as access to TB vaccines, which were constrained in 2026.
The only widely available TB vaccine is the Bacillus Calmette-Guérin (BCG), which has been in use since 1921.
Annually, hundreds of millions of doses are administered worldwide, and there are over 10 strain variants from different manufacturers. The WHO recommends administering BCG at birth in high-incidence countries for protection against severe childhood forms of TB, such as TB meningitis.
However, it provides limited or no protection against pulmonary TB.
In 2026, various BCG versions continue to be routinely offered in approximately 156 out of 194 countries with moderate to high TB incidence. Recently, no new preventive TB vaccine has been licensed for broad use.
Nonetheless, the pipeline looks promising, as of early 2026, with at least 17 vaccine candidates in clinical development, including six in Phase III trials (e.g., M72/AS01E, MTBVAC, VPM1002, GamTBvac, Immuvac/MIP, and others).
In the U.S., the TICE® BCG strain, produced by Merck (Organon Teknika Corp), has been the sole manufacturer since 2012. It is FDA-approved primarily for intravesical use in the treatment and prophylaxis of carcinoma in situ of the urinary bladder and for the prophylaxis of certain papillary tumors following transurethral resection. It is not routinely recommended for general TB prevention in the U.S.
The CDC considers BCG vaccination only in specific high-risk situations, such as for children continuously exposed to untreated or ineffectively treated TB cases when separation is not feasible.
In February 2026, access to TICE® BCG remains limited due to ongoing global demand outpacing supply.
To address the persistent shortage of the TICE® BCG vaccine, the FDA authorized an Expanded Access Program in February 2025 for recombinant BCG (rBCG), specifically TUBERVAC-rBCG, developed by the Serum Institute of India in partnership with ImmunityBio.
As of early 2026, this rBCG vaccine is offered at about 57 urology centers nationwide, enrolling patients and administering doses.
But the rBCG vaccine is not used for TB prevention.
As we celebrate World TB Day on March 24, 2026, ending TB outbreaks remains the goal!
The WHO says World TB Day focuses on ending this vaccine-preventable disease, but challenges remain in 2026.
Our Trust Standards: Medical Advisory Committee