100 Year Old Tuberculosis Vaccine Getting an Upgrade

After 100 years of use and billions of vaccinations, the world's deadliest infectious disease vaccine may soon be enhanced to meet the needs in 2025.
In a press release published on February 26, 2025, IAVI and Biofabri, a subsidiary of Zendal, announced that the first doses of tuberculosis MTBVAC vaccine candidate had been administered in the IMAGINE phase 2 clinical trial in Paarl, South Africa.
The IMAGINE trial will assess the safety and efficacy of a single-dose MTBVAC in preventing active tuberculosis (TB) lung disease in adolescents and adults, an underserved age group. Study participants will be followed for two to three years to assess the vaccine candidate's efficacy.
If MTBVAC is shown to be efficacious, Biofabri, IAVI, and other partners will collaborate to ensure a sufficient, affordable supply for low—and middle-income countries.
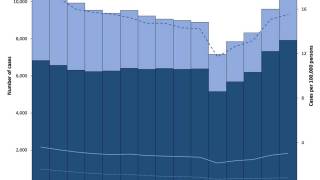
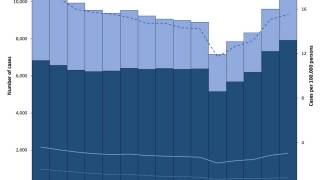
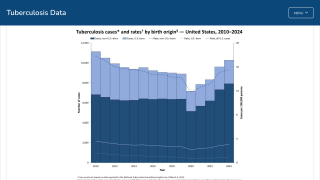
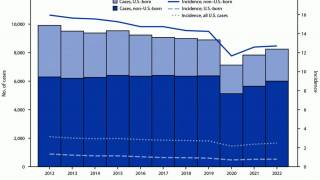
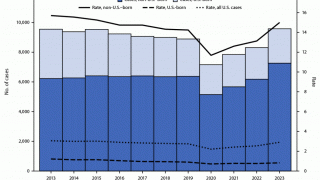
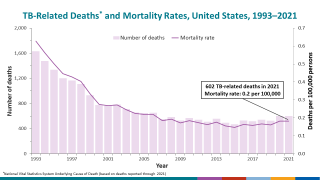
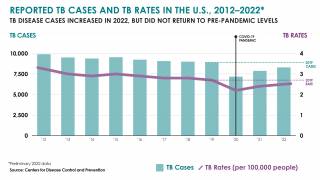
TB disease caused 1.25 million deaths in 2023, with cases reported in most countries, including an increasing number in the United States in 2024.
The only U.S. FDA-approved TB vaccine, Merck's Bacillus Calmette-Guérin (BCG) vaccine, offers partial protection (about 50%) to children but limited TB disease prevention in adolescents or adults.
IAVI wrote, 'A TB vaccine effective in children, adolescents, and adults would save millions of lives otherwise lost to TB over time.'
"We are working towards the same common goal: the end of the devastating impacts of TB disease. We are honored to be part of this coalition to ensure an accelerated R&D push for a new TB vaccine and its equitable, affordable global distribution once authorized," said Ana Céspedes, Pharm.D., MBA, and COO of IAVI, in the press release.
As of February 2025, about 15 versions of the BCG vaccine are in use globally, including in the U.S., which offers limited commercial access to the general public.
Recently, the FDA authorized importing the Serum Institute of India's TUBERVAC BCG vaccine to treat non-muscle-invasive bladder cancer patients.
Our Trust Standards: Medical Advisory Committee