BCG Vaccination Provides Additional Protections for Infants
A recent study published by the BMJ concluded that in newborn babies weighing <2000 g in intensive care, a dose of the Bacille Calmette-Guérin (BCG) Danish vaccine and oral polio vaccine reduced all-cause neonatal mortality owing to a decrease in deaths due to infections other than tuberculosis.
These researchers wrote on September 16, 2025, that a substantial reduction in neonatal mortality could be achieved if a skilled administrator vaccinated a high proportion of newborn babies in high mortality settings on the day of, or soon after, birth.
A multicentre, open-label, randomised controlled trial in India found that the BCG vaccine may have non-specific (off-target) effects, reducing the severity of other infections as well as tuberculosis.
These effects are due, at least in part, to heterologous immunity and "trained" innate immune responses mediated by epigenetic reprogramming of monocytes.
The beneficial, non-specific effects of BCG vaccination could last at least 1-2 years, depending on the BCG strain, and appear to be enhanced if there is existing immunity, such as a maternal BCG scar or BCG revaccination, as noted by these researchers.
According to the World Health Organization (WHO), versions of the BCG vaccine have been in use for approximately 100 years. Since 1921, over 4 billion BCG vaccinations have been completed worldwide. In 2023, 323 million doses of BCG were required worldwide.
The most commonly used strains were those prequalified by the WHO, such as Danish 1331, Bulgarian SL-222, and Tokyo 172-1.
As of October 2025, the WHO recommends BCG vaccination at birth in countries where tuberculosis is endemic.
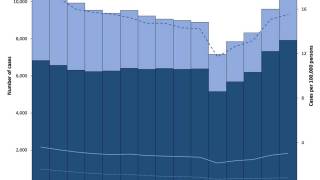
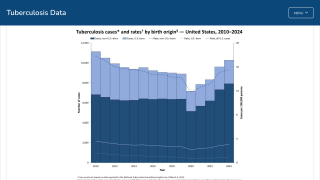
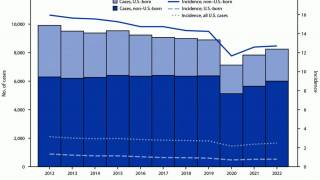
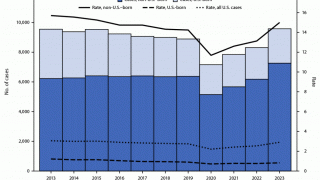
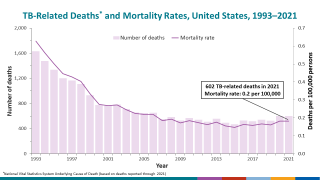
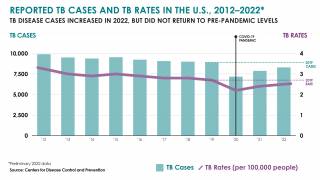
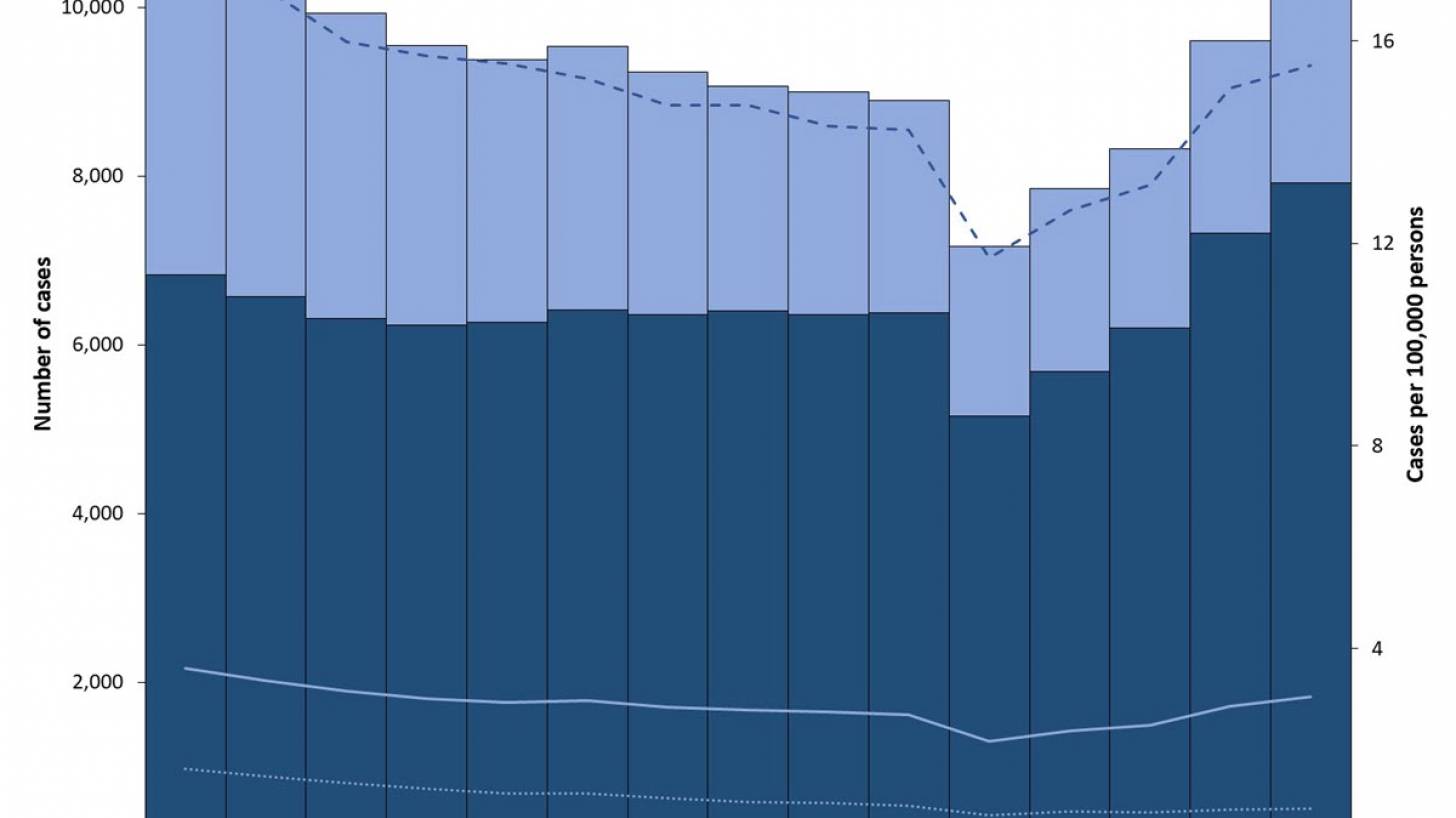
After nearly three decades of decline in the United States, the number of tuberculosis cases began increasing in 2021. Since then, four states have been the unfortunate leaders in TB cases: California, Florida, New York, and Texas.
Merck's TICE® BCG vaccine is approved by the U.S. Food and Drug Administration (STN: 103050), but is not widely used in the United States.
However, BCG vaccination is available in certain health situations, such as for bladder cancer.
Our Trust Standards: Medical Advisory Committee