India's Novel Polio Vaccine Gains WHO Prequalification
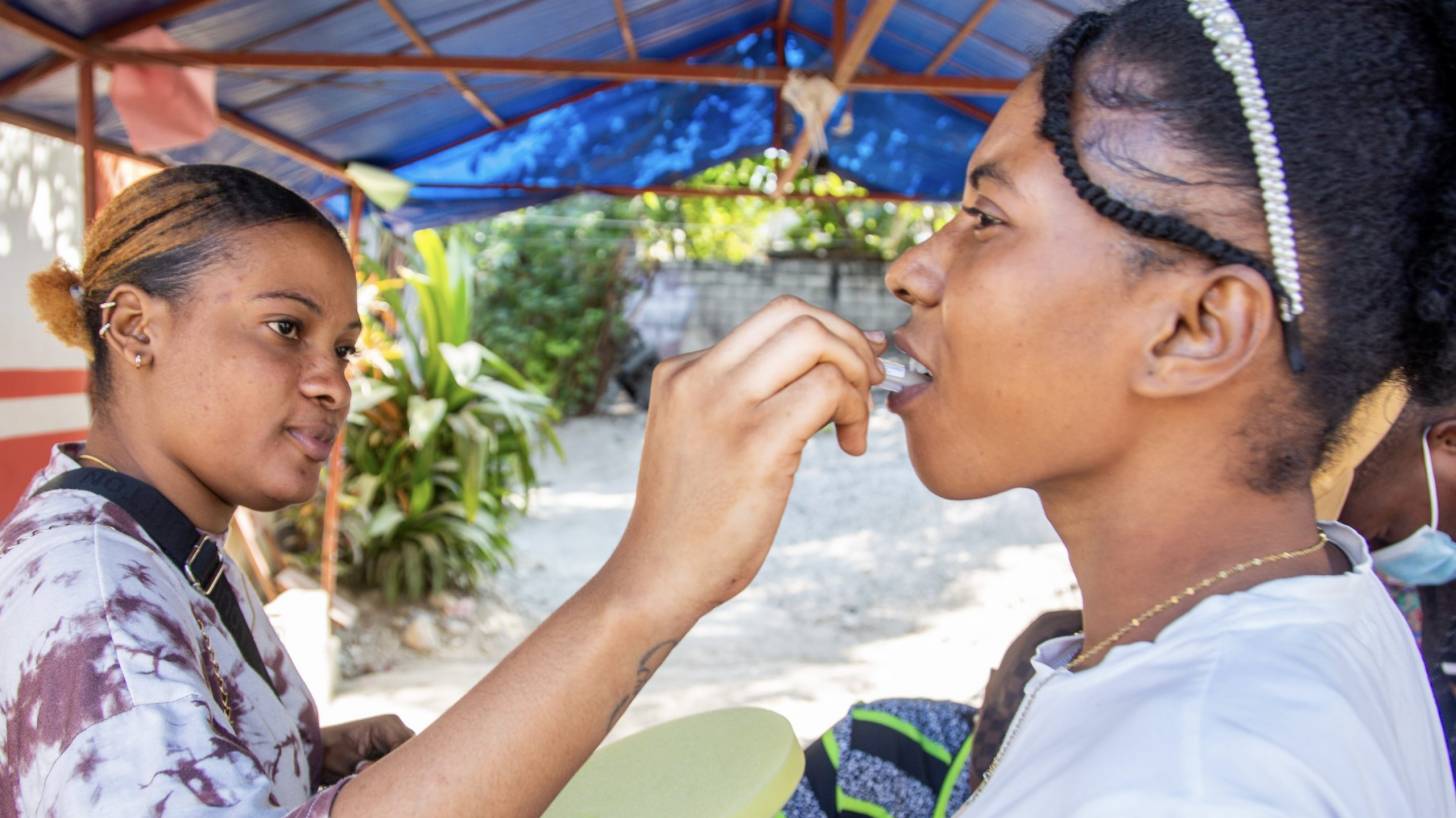
In a significant milestone for global health, the Global Polio Eradication Initiative (GPEI) recently welcomed the World Health Organization's (WHO) decision to grant prequalification (PQ) status to the novel oral polio vaccine type 2 (nOPV2) produced by India's Biological E. Limited (BE).
Announced on February 12, 2026, this approval expands BE's role from fill-finish activities using bulk vaccine supplied by PT Bio Farma (PTB) in Indonesia to full-scale manufacturing, bolstering the global supply chain for this essential tool in combating poliovirus outbreaks.
Since March 2024, BE has contributed 700 million doses of nOPV2 to the global stockpile, and with the new PQ, the company is poised to produce an additional 600 million doses annually.
The addition of BE as a manufacturer underscores India's leadership in vaccine production for the Global South, ensuring affordable and timely access.
This development positions BE as the second full manufacturer of nOPV2 alongside PTB, enhancing diversified and stable production capacity to address outbreaks of type 2 variant poliovirus (cVDPV2) more effectively.
This next-generation vaccine modifies the Sabin type 2 strain with genetic "locks" to enhance stability, reducing the risk of reversion to neurovirulence while maintaining immunogenicity and ease of administration.
Field rollout began in March 2021, initially under strict monitoring to confirm its genetic stability. Full WHO prequalification followed in December 2023 for PTB's version, paving the way for broader access.
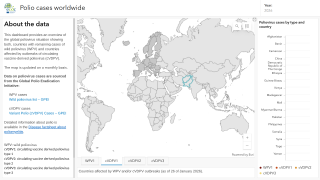
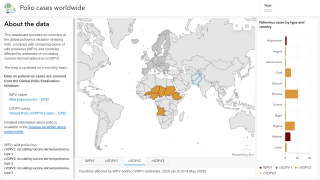
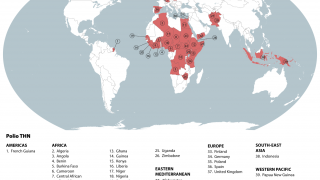
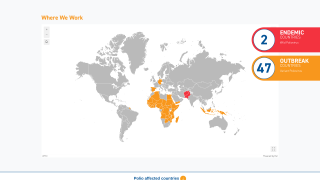
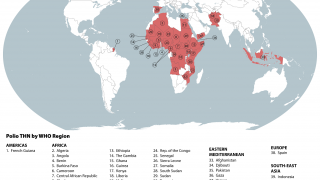
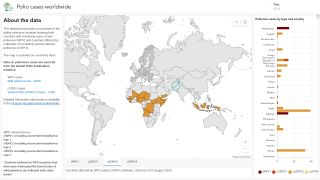
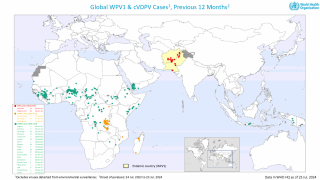
By 2026, over 2 billion doses of nOPV2 have been administered across more than 35 countries, primarily in Africa and Asia, where cVDPV2 outbreaks remain a threat.
Despite rare instances of reversion, such as a neurovirulent strain detected in Uganda in 2025 that did not spread widely due to high vaccination coverage, nOPV2's stability has been a game-changer.
According to the GPEI, nOPV2 is indispensable for rapid outbreak response, complementing IPV in routine schedules to provide both individual protection and community immunity.
The GPEI's 2022-2026 strategy emphasizes its role in interrupting transmission, and experts note that prematurely withdrawing OPV could reverse decades of progress, potentially leading to millions of cases of paralysis.
Dr. Kathleen Neuzil, Director for Polio at the Gates Foundation, added in a press release, "As a next-generation vaccine, nOPV2 is a major scientific advance for polio outbreak response. Having Biological E as a full manufacturer expands the dependable supply countries need to protect children and sustain progress toward eradication."
As of February 20, 2026, the nOPV2 has been deployed in over 40 countries but is unavailable in the United States; the IPV vaccine has been offered since 2000.
Our Trust Standards: Medical Advisory Committee