Vaccinations Can Reduce Wild Poliovirus Cases
Since the World Health Organization (WHO) reconfirmed that the spread of the poliovirus remained a Public Health Emergency of International Concern in April 2025, various initiatives have been launched to advance vaccination efforts.
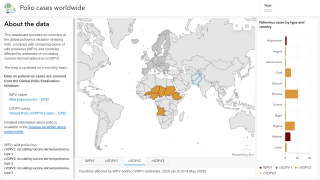
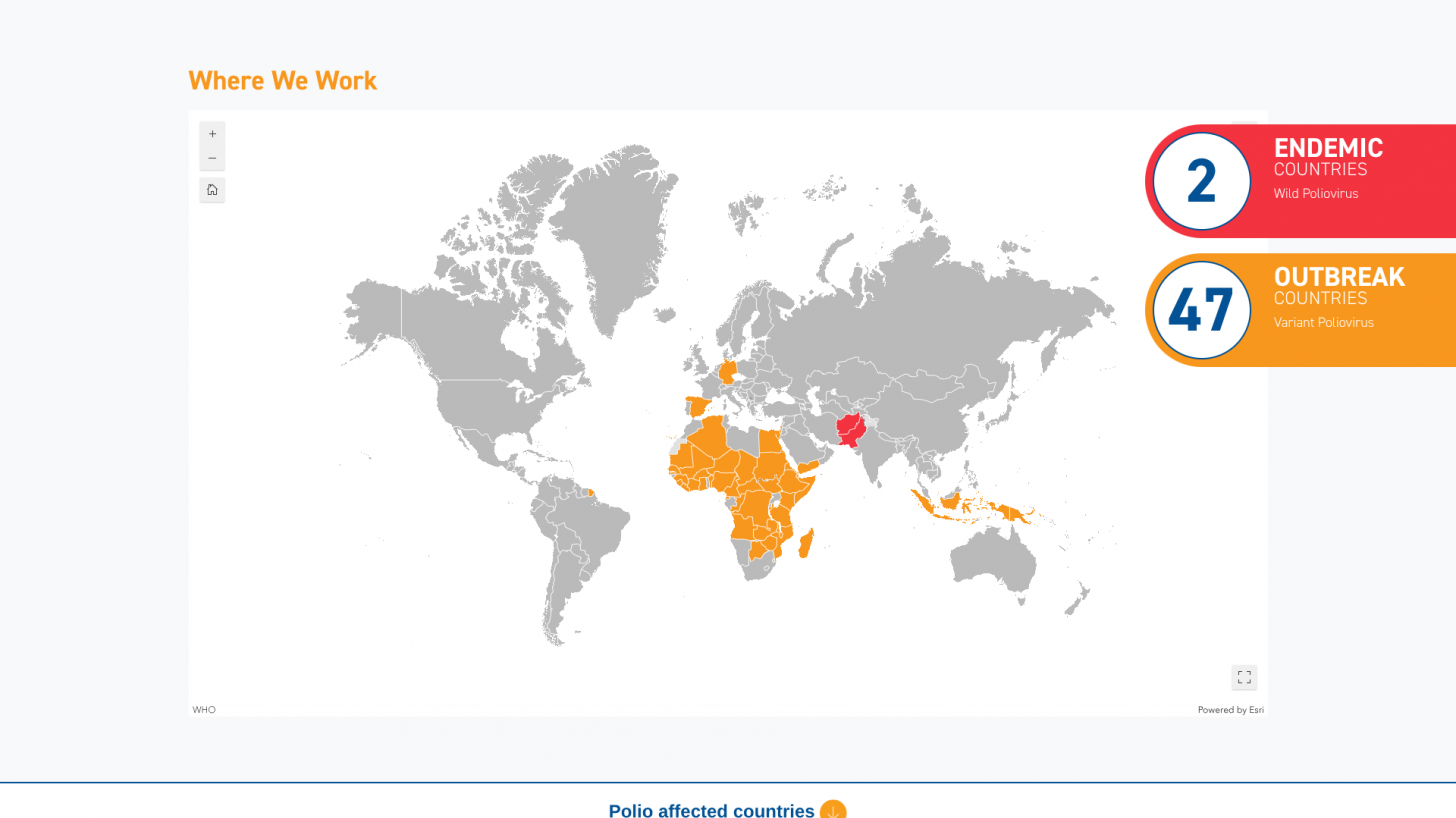
The WHO's Strategic Advisory Group of Experts on Immunization report, published on June 6, 2025, highlights concerns over the rising number of wild virus cases and the spread of variant poliovirus into new regions.
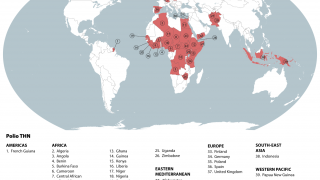
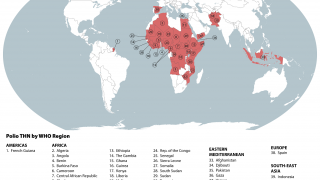
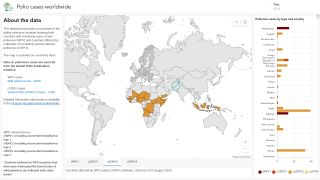
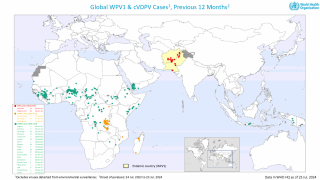
Just last week, the Global Polio Eradication Initiative confirmed six countries had reported cases of wild poliovirus type 1. Papua New Guinea recently reported that acute flaccid paralysis cases have been reported across 11 provinces.
As requested by SAGE, the report focused on the key roles of WHO in delivering on priority areas of immunization progress to reach the Immunization Agenda 2030 goals.
For example, the WHO supports bOPV cessation plans, endorses a new risk-assessment framework for IPV-only schedules, and updates key vaccine recommendations.
The bOPV is being replaced with the type 2 novel oral polio (nOPV2) vaccine, which has been 'triple-locked' using genetic engineering to prevent it from becoming harmful and producing a mutation.
As a result, nOPV2 is reported to be more genetically stable than previous OPV, with a lower risk of reversion to neurovirulence and a reduced likelihood of mutation, which can cause paralysis.
In the United States, the IPV vaccine has been exclusively deployed since 2000.
The U.S. Centers for Disease Control and Prevention's (CDC) Advisory Committee on Immunization Practices (ACIP) reviewed and voted on updated guidance for IPV vaccination in June 2023.
The ACIP reviewed a presentation on October 19, 2022, that confirmed IPV induces some nasopharyngeal mucosal immunity but limited intestinal immunity.
However, IPV induces effective humoral immunity and prevents paralysis in approximately 90% of cases after two doses of the vaccine. Efficacy is 99% after 3 doses.
Currently, the CDC advises that children should get an IPV to protect against poliomyelitis, according to the 2025 vaccination schedule.
Additionally, the CDC recommends that children traveling to countries with a risk of contracting poliovirus complete the series before departure.
Most adults do not need a polio vaccine booster because they received the vaccine as children, unless they are visiting areas with ongoing polio outbreaks. In that situation, the CDC endorses one booster dose for added protection.
Polio vaccination services are offered at travel clinics and pharmacies in the U.S.
Our Trust Standards: Medical Advisory Committee