Lyme Disease Vaccine Candidate Delivers Strong Immune Response Following Third Yearly Booster Dose
The Lancet Infectious Diseases recently published results from a phase 2 clinical trial of the Lyme disease vaccine candidate, VLA15.
The Valneva SE and Pfizer Inc.-funded study showed a strong immune response following a third booster dose, with a favorable safety profile.
These researchers wrote on November 7, 2025, that the results are consistent with those observed following previous annual booster doses, further supporting the expected benefits of a yearly vaccination before each Lyme season.
They concluded, 'The safety and robust anamnestic immune responses associated with VLA15 boosting support its use as a strategy to increase anti-OspA antibody levels before tick season among children, adolescents, and adults.'
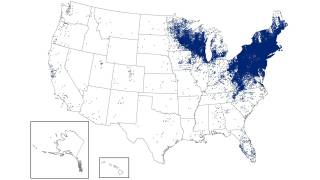
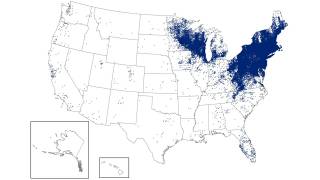
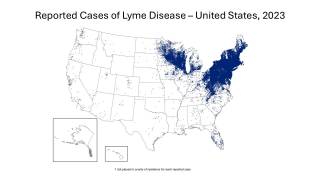
This study's findings are essential to public health as the the Centers for Disease Control and Prevention estimates that approximately 476,000 people in the U.S. are diagnosed and treated for Lyme disease each year, and 132,000 cases are reported annually in Europe.
As of November 14, 2025, VLA15 is the most advanced Lyme disease vaccine candidate.
Our Trust Standards: Medical Advisory Committee