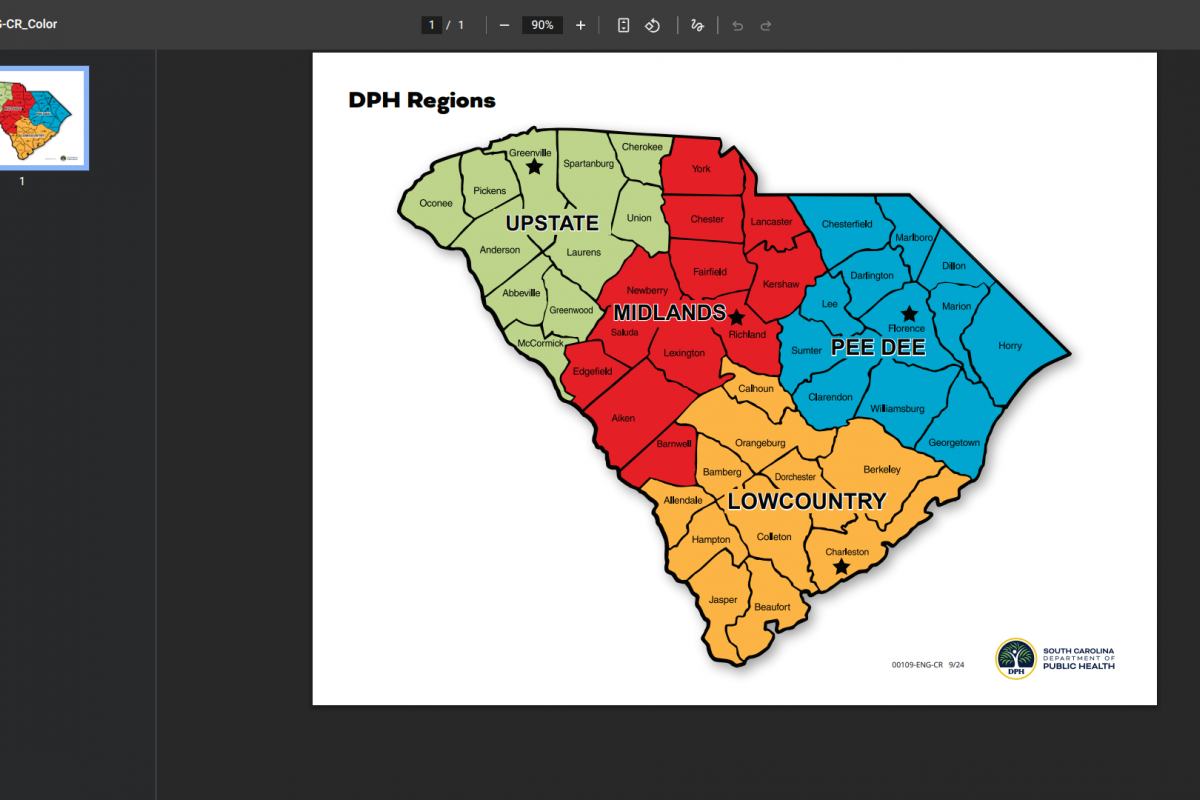
The South Carolina Department of Public Health (DPH) recently confirmed an outbreak of measles in the Upstate area.
As of October 1, 2025, a total of eight measles cases have been reported. Five out of the eight instances became sick within the past month.
Currently, cases are following DPH isolation guidance to prevent further spread of the vaccine-preventable virus near Clemson University, which has a student population exceeding 7,000.
"Measles is highly contagious, and there is risk for continued, rapid spread of the disease in the Upstate among communities with low immunization rates," said Dr. Linda Bell, state epidemiologist and Health Programs Branch director, in a press release.
"Measles-mumps-rubella (MMR) vaccination remains the most important tool for preventing measles infection and spread. We strongly encourage everyone to review their immunization records and make sure they are up to date on all recommended vaccinations, including the MMR."
"The unknown source of two of the cases indicates unrecognized community spread," said Dr. Bell. "We anticipate more cases will be identified and implore community members to act responsibly. If you are ill, stay home."
South Carolina is not alone in reporting measles outbreaks.
As of September 2025, over 1,500 confirmed measles cases have been reported across 41 states, according to the Centers for Disease Control and Prevention data. The majority of measles cases were reported in Texas.
Additionally, both Canada and Mexico continue to report measles outbreaks in 2025.