Fractional IPV Doses Endorsed to Eradicate Polio
The World Health Organization's Strategic Advisory Group of Experts on Immunization (SAGE) recently endorsed two critical innovations for polio eradication. SAGE strongly emphasized that polio eradication cannot be achieved solely through technical interventions.
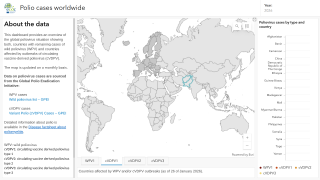
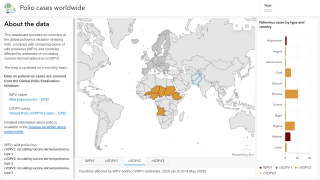
As of the end of September 2025, the SAGE recommended that fractional doses of Sabin-based inactivated polio vaccine (IPV) be used in the same way as fractional doses of Salk-based IPV.
This recommendation should stretch the vaccine supply and reach more children.
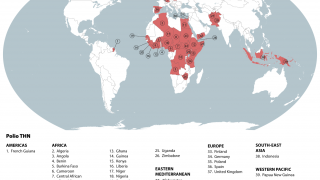
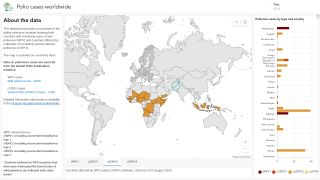
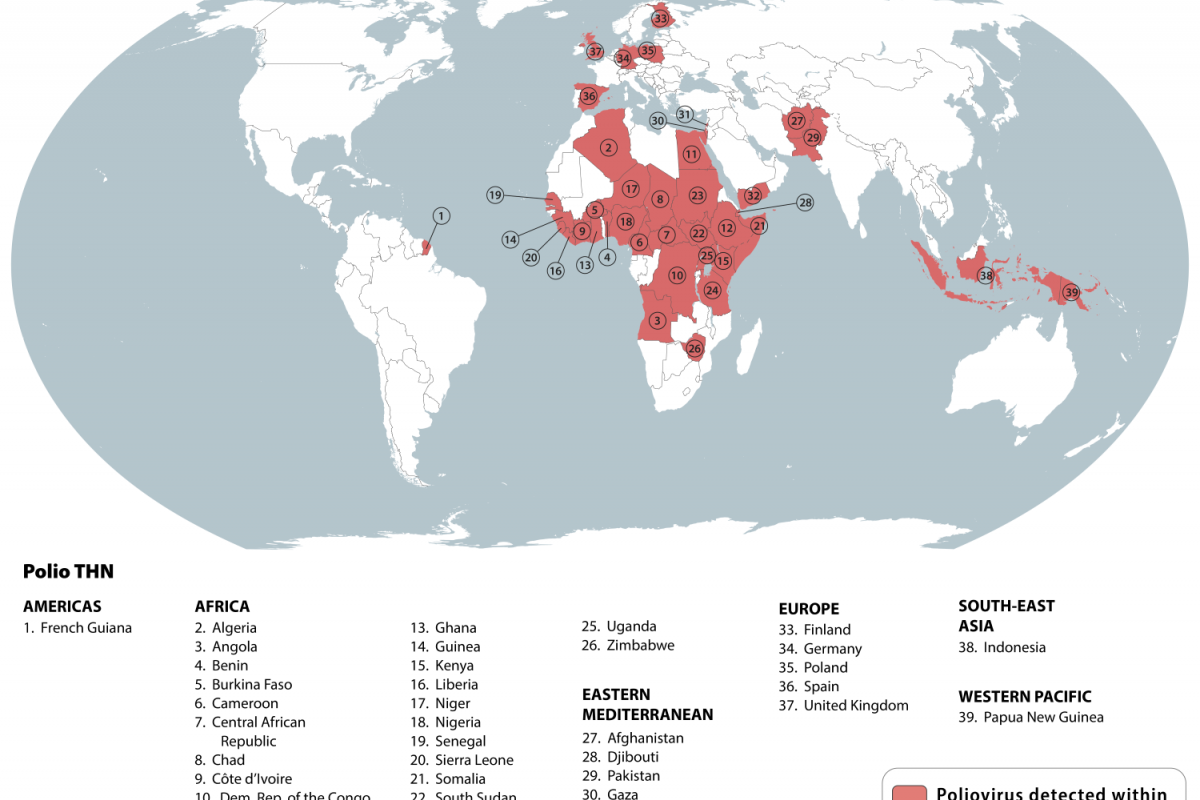
SAGE also supported the broader rollout of novel oral polio vaccine type 2 (nOPV2) to help prevent persistent outbreaks of circulating variant poliovirus type 2 (cVDPV2) in some of the most challenging areas.
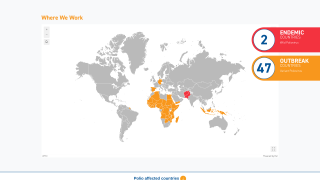
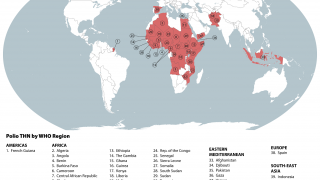
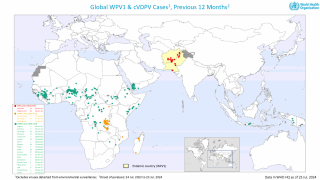
SAGE stated it is very concerned about the continued transmission of wild poliovirus (WPV1) in Pakistan and Afghanistan.
Last week, Pakistan reported three cases of WPV1.
While improving routine immunization coverage, vaccination campaigns, surveillance, and outbreak response remain essential, the decisive factor is sustained national political leadership and accountability at every level, added SAGE.
In the United States, the IPV remains the only polio vaccine offered at clinics and pharmacies.
The U.S. CDC states that before traveling to any at-risk destination, adults who have previously completed the whole, routine polio vaccine series may receive a single, lifetime booster dose of the IPV polio vaccine. Polio vaccination services are offered at clinics and pharmacies in the USA.
Our Trust Standards: Medical Advisory Committee