16th Ebola Outbreak Declared in Africa
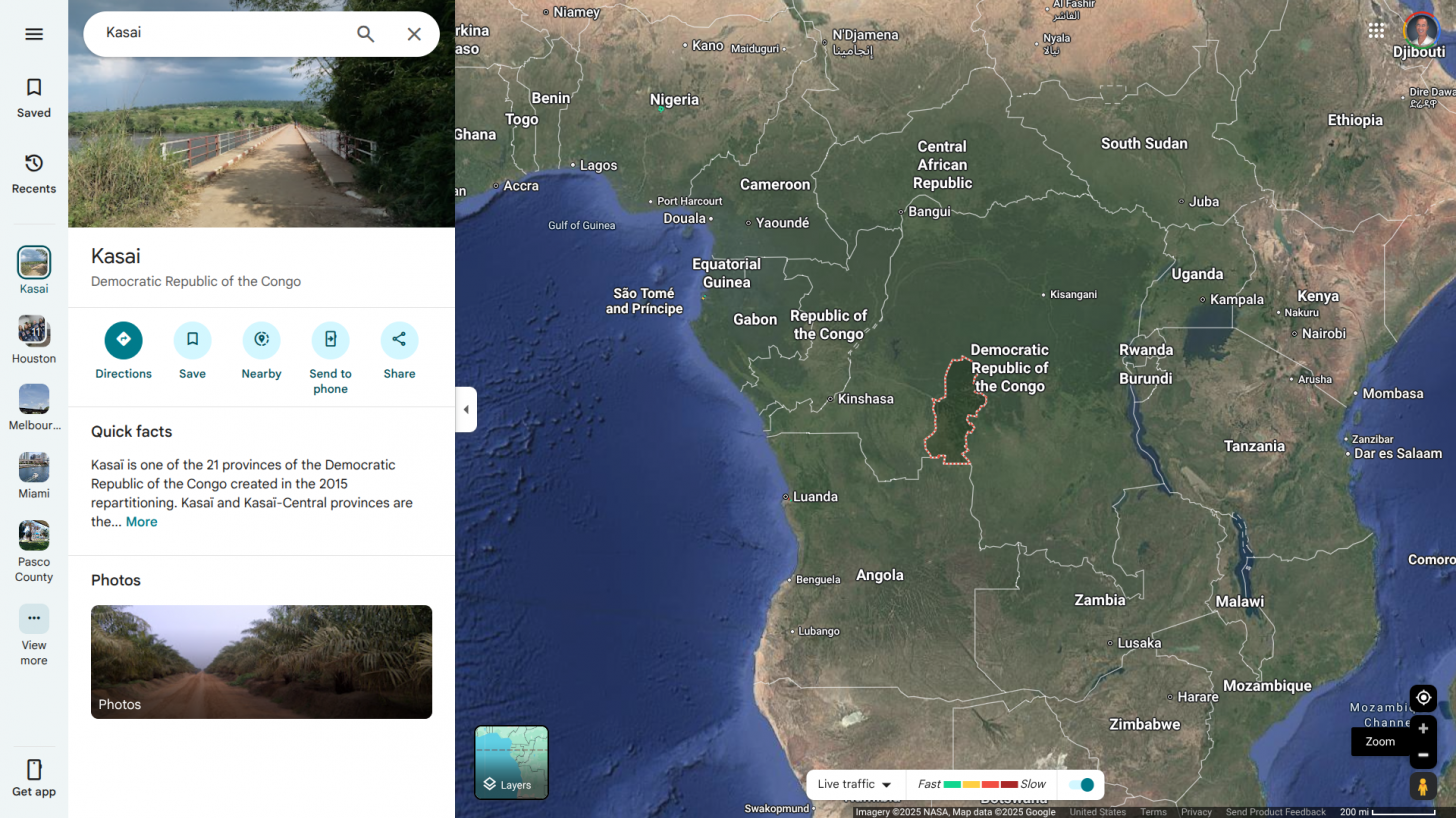
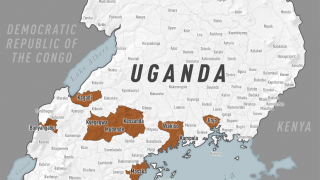
Health authorities in the Democratic Republic of the Congo (DRC) today declared a new outbreak of Ebola Zaire virus disease in Kasai Province.
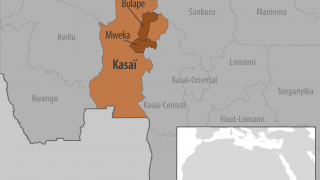
As of September 4, 2025, this outbreak has infected 28 suspected cases and resulted in 15 deaths, including four health workers, in the Bulape and Mweka health zones of Kasai Province.
Case numbers are likely to increase as transmission continues, says the World Health Organization (WHO).
The WHO states that this area in the DRC is difficult to access, requiring at least one day of driving from Tshikapa, the capital of Kasai.
A national Rapid Response Team, joined by WHO experts in epidemiology, infection prevention and control, laboratory, and case management, has been deployed to Kasai Province.
Additionally, WHO is delivering two tonnes of supplies, including personal protective equipment, mobile laboratory equipment, and medical supplies.
The DRC has a stockpile of treatments, as well as 2,000 doses of the Ervebo Ebola vaccine, already prepositioned in Kinshasa.
"We're acting with determination to halt the spread of the virus and protect communities rapidly," said Dr Mohamed Janabi, WHO Regional Director for Africa, in a media release.
"Banking on the country's long-standing expertise in controlling viral disease outbreaks, we're working closely with the health authorities to quickly scale up key response measures to end the outbreak as soon as possible."
The DRC's last outbreak of Ebola virus disease affected the north-western Equateur province in April 2022. It was brought under control within three months, thanks to the robust efforts of the health authorities.
In Kasai province, previous outbreaks of Ebola virus disease were reported in 2007 and 2008.
The WHO states Ebola virus disease is a rare but severe, often fatal illness in humans. However, there have been numerious outbreaks in the DRC since the disease was first identified in Africa during 1976.
It is transmitted to people through close contact with the blood, secretions, organs, or other bodily fluids of infected animals such as fruit bats (thought to be the natural hosts). Human-to-human transmission is through direct contact with the blood or body fluids of a person who is sick with or has died from Ebola. These objects have been contaminated with body fluids from a person sick with Ebola or the body of a person who died from Ebola.
During previous outbreaks, air passengers from outbreak areas were screened for the virus at airports in the United States.
To accelerate Ebola virus testing, Aptitude Medical Systems announced in July 2025 its second partnership with the Biomedical Advanced Research and Development Authority (BARDA). This collaboration leverages Aptitude's next-generation molecular diagnostics platform, Metrix®, which has been advanced through a prior BARDA partnership valued at up to $61.9 million.
This rapid next-generation molecular diagnostic device aims to detect and differentiate Ebolavirus and Marburgvirus species.
As of September 4, 2025, Ebola vaccines are not commercailly available in the U.S.
Our Trust Standards: Medical Advisory Committee