Rapid Diagnostic Tests for Ebola and Marburg Viruses Received $9 Million

When Ebola and Marburg outbreaks have occurred over the decades, diagnosing cases has been a significant challenge for healthcare workers.
To address this essential need, Aptitude Medical Systems announced its second major partnership with the Biomedical Advanced Research and Development Authority (BARDA), with $9 million in funding to develop the Metrix Filovirus Panel.
This collaboration leverages Aptitude's next-generation molecular diagnostics platform, Metrix®, which has been advanced through a prior BARDA partnership valued at up to $61.9 million.
This rapid next-generation molecular diagnostic device aims to detect and differentiate Ebolavirus and Marburgvirus species.
"Point-of-care diagnostics are essential for effectively addressing outbreaks of high-consequence pathogens like Ebolavirus and Marburgvirus species," added JP Wang, PhD, CTO, President, and Executive Chairman of Aptitude, in a press release on July 28, 2025.
It is a small, portable platform, making it appropriate for use in remote and more traditional point-of-care settings, generating results in 30 minutes or less from venous or fingerstick blood samples.
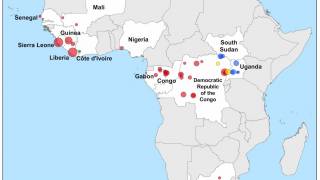
As of July 29, 2025, there are no active Ebola and Marburg outbreaks in Africa, and Ebola vaccines and antibody therapies have been approved for use by various countries.
Our Trust Standards: Medical Advisory Committee