Singapore Launches Third-Generation Dengue Vaccine Study
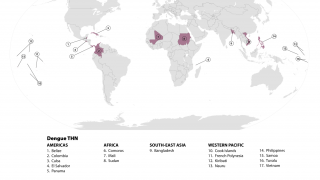
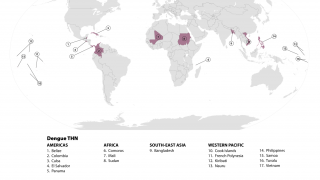
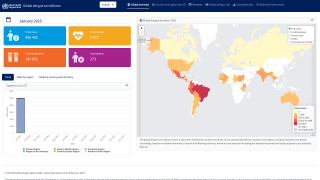
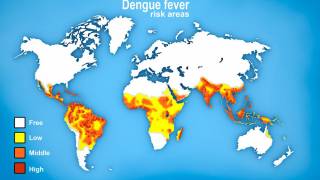
According to a recent World Health Organization report, the Western Pacific Region is facing significant dengue outbreaks for the second consecutive year.
Since dengue is a vaccine-preventable disease, countries such as the Republic of Singapore are conducting clinical trials pursuing the use of a third-generation vaccine.
On June 18, 2025, MSD (Merck & Co.) announced the initiation of the MOBILIZE-1 Phase 3 clinical trial (V181-005) evaluating the safety, immunogenicity and efficacy of a single dose of V181, a quadrivalent vaccine candiate, for the prevention of dengue disease caused by any of the four dengue virus serotypes, regardless of prior dengue exposure.
Recruitment for this late-stage trial has begun, and the first participants are now enrolling in Singapore.
The study is planned to include more than 30 trial sites in dengue-endemic areas in the Asia-Pacific region, including Indonesia, Malaysia, the Philippines, Singapore, Thailand, and Vietnam.
Dr. Abdullahi Sheriff, Managing Director, MSD in Singapore, Malaysia & Brunei, commented in a press release, “We are grateful to the scientific community in Singapore for their commitment to scientific excellence and partnership throughout this journey."
"Singapore and the surrounding countries in Southeast Asia are areas with a risk for dengue, making it a serious public health threat."
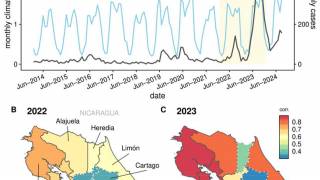
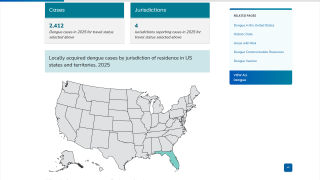
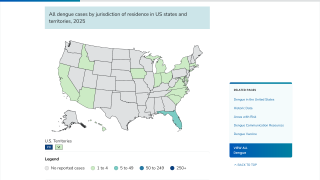
Throughout 2024 and during the first half of 2025, dengue cases and related fatalities have been reported in most countries in the Region of the Americas, including the United States.
The global need for additional dengue vaccines is substantial. As of June 20, 2025, the second-generation QDENGA vaccine is in limited supply worldwide and is unavailable in the U.S.
Our Trust Standards: Medical Advisory Committee