Europe recommends Access to Innovative Bladder Cancer Therapy
Across the European Union, Bladder cancer is a serious public health concern, ranking as the fifth-most common cancer. Specifically, each year, more than 150,000 people in Europe are diagnosed with non-muscle invasive bladder cancer (NMIBC).
Based on a recommendation from the European Medicines Agency (EMA), early access to an innovative therapy targeting this condition will be available.
California-based ImmunityBio Inc. announced on December 12, 2025, that the EMA had recommended granting a conditional marketing authorization in the EU for ANKTIVA® in combination with Bacillus Calmette-Guérin (BCG) for the treatment of BCG-unresponsive NMIBC carcinoma in situ.
ImmunityBio says its BioShield platform, powered by Anktiva®, represents a paradigm shift in cancer care.
"ANKTIVA offers a new treatment option for patients and addresses an important unmet need," the EMA noted in an announcement on the recommendation. "There are currently no authorised treatments for NMIBC that do not respond to BCG."
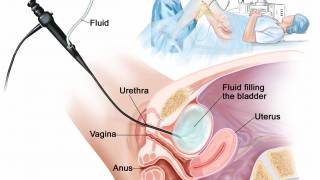
The EMA wrote that Anktiva will be available as a 400 μg concentrate for intravesical suspension. Anktiva is an interleukin-15 (IL-15) receptor agonist that enhances immune responses by stimulating CD4+ and CD8+ T cells, as well as NK cells.
The benefits of Anktiva are a 71% (95% CI: 61.1, 79.6) complete response rate and 26.6 months (95% CI: 13.0, 49.9) median duration of complete response in adults with BCG-unresponsive NMIBC with CIS, with or without papillary tumours, in a single-arm, open-label, phase 2/3 study.
The most common side effects with Anktiva are dysuria, haematuria, pollakiuria, urinary tract infection, micturition urgency, fatigue, chills, musculoskeletal pain, and pyrexia, says the EMA.
"ANKTIVA represents an important evolution in the treatment of NMIBC CIS, strengthening the immune response and improving the durability of BCG," said Dr. Patrick Soon-Shiong, Founder, Executive Chairman, and Global Chief Scientific and Medical Officer of ImmunityBio, in a press release.
"Hundreds of patients in the U.S. are already experiencing the benefits of this therapy, and our goal is to make it available to patients in Europe and other parts of the world as quickly and responsibly as possible, to ensure avoidance of a radical cystectomy."
"We are pleased that the EMA issued this positive recommendation based on our single-arm trial and through a regulatory process that allows earlier access to ANKTIVA, as stated in the EMA announcement, the benefit of a medicine's immediate availability to patients outweighs the inherent risks."
While the BCG vaccine has been used for about 100 years to prevent tuberculosis, it's also been an optional therapy for bladder cancer patients in the United States. Since 1921, more than 4 billion BCG vaccinations have been administered worldwide.
However, access to BCG in December 2025 is constrained.
According to the EMA, a BCG access solution has been identified in Europe.
"Six BCG strains are available in Europe for use in combination with ANKTIVA, and we are expeditiously developing our recombinant BCG candidate to address ongoing BCG shortages in the U.S. and help ensure that all eligible patients can benefit from this treatment," said Richard Adcock, President and CEO of ImmunityBio.
As of December 20, 2025, there are two types of BCG vaccines (TICE® BCG and recombinant BCG) approved by the U.S. FDA.
Worldwide, over 10 versions of the BCG vaccine are currently in use.
Our Trust Standards: Medical Advisory Committee