BCG Vaccine Shortage Ends in the United States

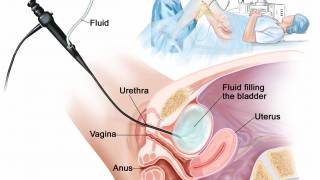
Following years of inadequate supply, a recently approved recombinant Bacillus Calmette-Guérin (rBCG) is now available in the United States to treat non-muscle invasive bladder cancer (NMIBC) patients.
Access to this vaccine may end the multi-year shortage of the TICE® BCG vaccine.
ImmunityBio, Inc. announced today that the U.S. Food and Drug Administration (FDA) has authorized an expanded access program that will bring an alternative source of BCG, a standard-of-care medicine in bladder cancer, to patients in the United States.
As of February 19, 2025, ImmunityBio says shipments of the Serum Institute of India (SII) TUBERVAC-rBCG vaccine will begin immediately in the U.S.
TUBERVAC-rBCG was approved in India in 2023 as a single-dose tuberculosis (TB) prevention vaccine. In European bladder cancer phase 2 clinical trials, the rBCG vaccine has demonstrated potent immunogenicity with CD8+ and CD4+ T cell stimulation and improved safety compared to earlier BCG strains and formulations.
"With the increasing threat of supply shortages of essential medicines, the biopharmaceutical industry must innovate and secure new means of ensuring uninterrupted access to vital therapeutics," said Dr. Patrick Soon-Shiong, Founder, Executive Chairman and Global Chief Scientific and Medical Officer of ImmunityBio, in a press release.
"Our collaboration with the FDA and SII to ensure a reliable supply of this vital drug for bladder cancer patients underscores ImmunityBio's commitment to addressing critical access issues that affect so many patients."
BCG is a benign bacterium that was developed over 100 years ago as a live vaccine against TB. It has been administered to more than 4 billion individuals worldwide. In 2002, Neonatal BCG vaccination was practiced in 157 countries and territories.
SII is the world's largest manufacturer of BCG vaccines, while Merck & Co. is currently the only manufacturer of the U.S. FDA-approved TICE® BCG vaccine.
The WHO's Civil Society Taskforce on TB confirmed that 16 TB vaccines were available globally. Two gene modifications have been implemented in rBCG to improve its immunogenicity and safety compared to earlier strains and formulations of BCG.
ImmunityBio's FDA-approved ANKTIVA® is a cytokine interleukin-15 that plays a crucial role in the immune system by affecting the development, maintenance, and function of key immune cells, NK and CD8+ killer T cells, that kill cancer cells. ANKTIVA's protocol integrates rBCG vaccinations.
Our Trust Standards: Medical Advisory Committee