Partnership to Produce 50 Million Dengue Vaccines Annually

Takeda and Biological E. Limited today announced a strategic partnership to accelerate access to QDENGA® (Dengue Tetravalent Vaccine [Live, Attenuated]) multi-dose vials (MDVs).
MDVs offer economic and logistical advantages for National Immunization Programs by minimizing packaging and storage expenses and reducing medical and environmental waste.
These doses will ultimately be made available for government procurement in dengue-endemic countries by 2030 to support National Immunization Programs.
"Takeda's long-term goal for our dengue program has been to make QDENGA broadly available to those at risk who may benefit from immunization. Within the last year, we've successfully launched in private markets, are now launching in some public programs, and working with partners to support a broader public health impact," said Gary Dubin, M.D., president of the Global Vaccine Business Unit at Takeda.
According to the press release on February 27, 2024, BE will ramp up to a manufacturing capacity of up to 50 million doses a year, accelerating Takeda's efforts to manufacture 100 million doses annually within the decade.
The partnership will build upon existing manufacturing capacity for the vaccine at Takeda's facility in Singen, Germany, and Takeda's long-term collaboration with IDT Biologika GmbH.
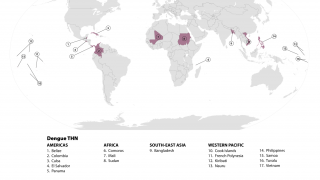
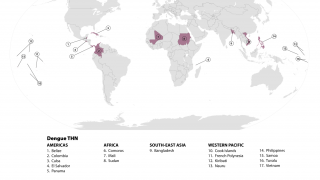
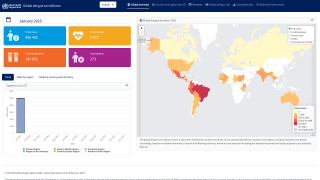
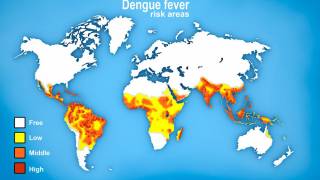
Dengue fever is among the most common mosquito-borne viral diseases worldwide. Dengue is endemic in more than 100 countries and causes an estimated 390 million infections yearly.
The Americas, Southeast Asia, and Western Pacific regions are the most seriously affected, with Asia alone representing ~70% of the global disease burden.
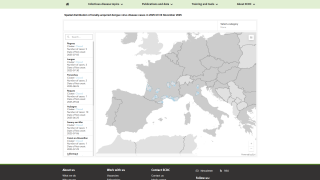
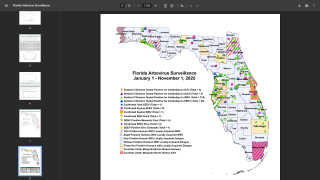
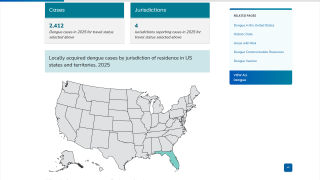
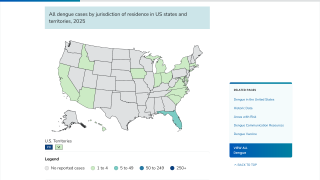
In 2024, the state of Florida reported two cases of locally acquired dengue from two counties. In 2023, positive samples from 186 humans were reported from five counties.
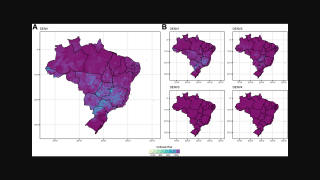
QDENGA is currently available in the private market in countries in Europe, Indonesia, and Thailand and in private and some public programs in Argentina and Brazil.
As of late February 2024, QDENGA is not approved by the U.S. FDA.
Our Trust Standards: Medical Advisory Committee