In Taiwan (Republic of China), Japanese Encephalitis (JE) has been a notifiable, mosquito-transmitted infectious disease since 1955.
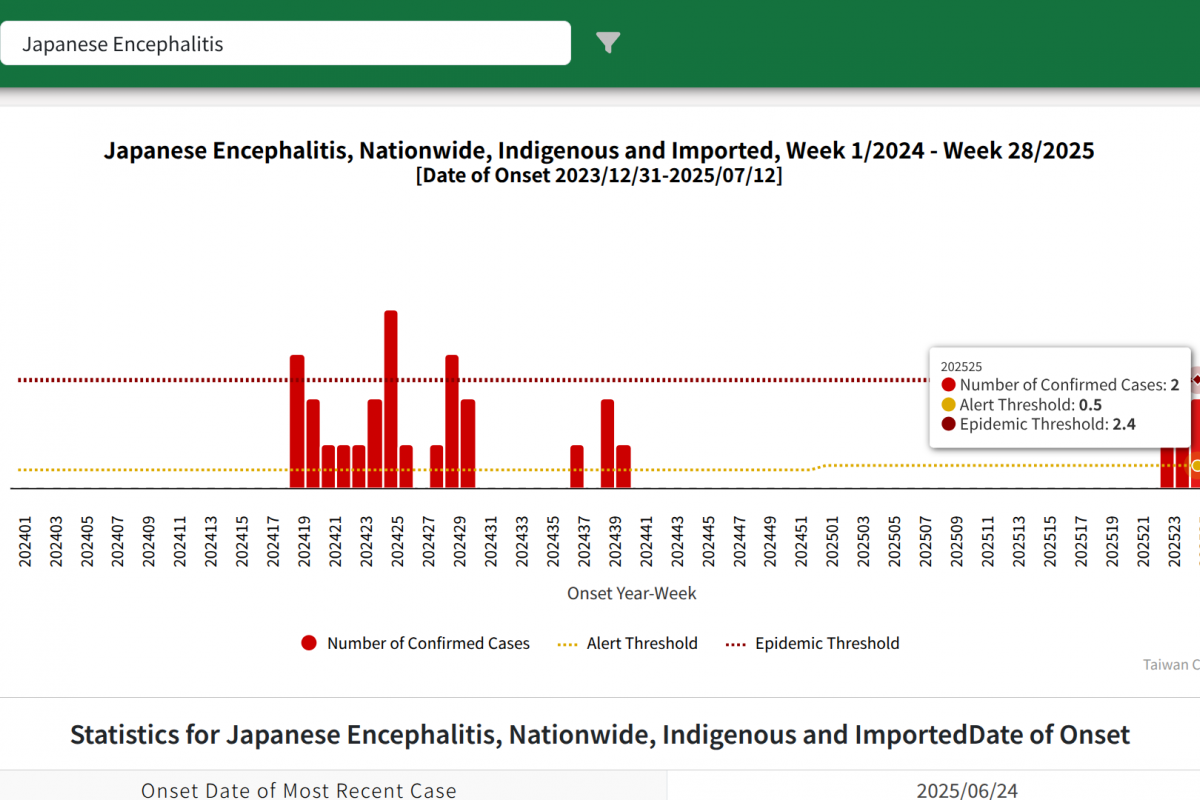
As of July 2025, the Taiwan Centers for Disease Control (TCDC) confirmed six cases of JE in 2025.
Last year, Taiwan reported 25 JE cases.
Most JE cases are associated with activities in paddy fields, pig farms, pigeon farms, poultry farms, and ponds.
A recent study confirmed that JE remains a prevalent infectious disease in Taiwan. Between 2008 and 2020, 309 confirmed domestic JE patients and four imported cases.
This study confirmed that JE remains a prevalent infectious disease in Taiwan, with its epidemic gradually increasing in severity.
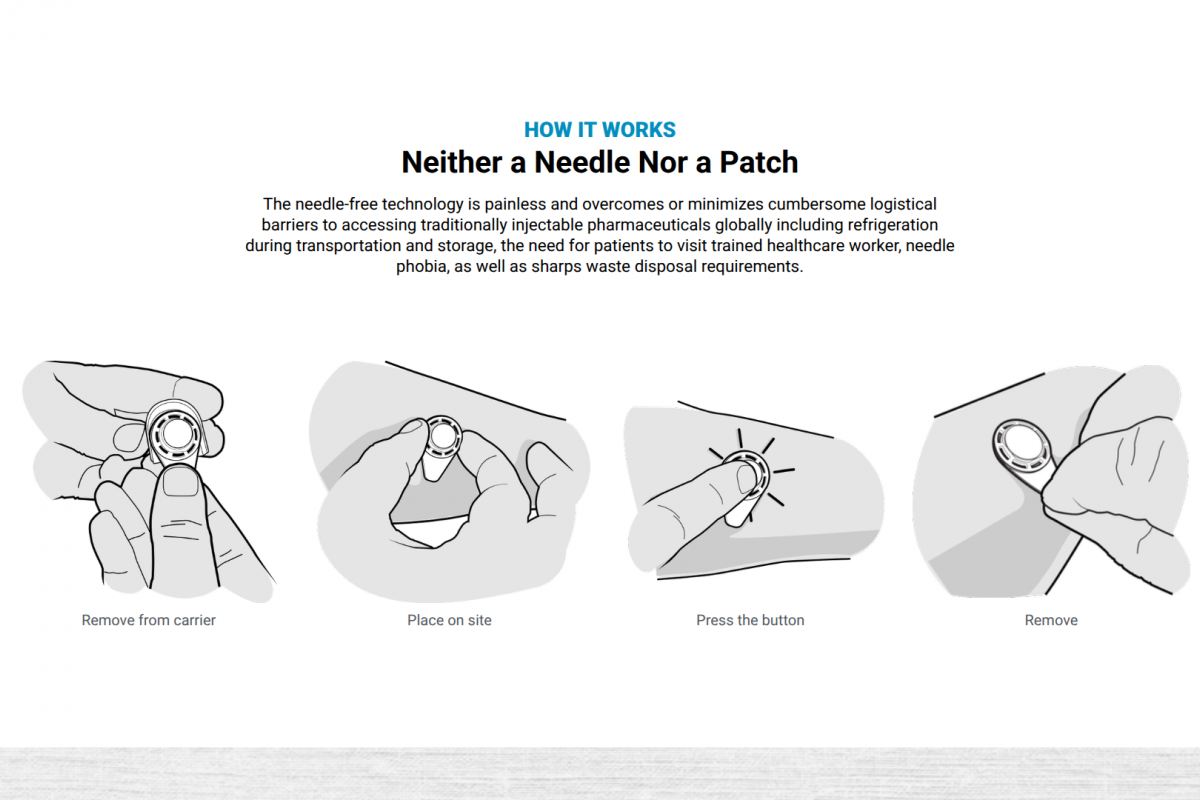
As of July 7, 2025, JE is a vaccine-preventable disease. In the United States, an FDA-approved JE vaccine is commercailly available at travel clinics and pharmacies. It is recommended for international travelers when visiting JE outbreak areas.