UK Approves Intravesical Therapy Targeting Bladder Cancer

An innovative bladder cancer intravesical therapy today gained its initial approval outside the United States. Bladder cancer is a challenging malignancy, and for many years, researchers have searched for next-generation treatment options.
The UK's Medicines and Healthcare products Regulatory Agency (MHRA) has approved nogapendekin alfa inbakicept (Anktiva®) for adults with BCG-unresponsive non-muscle-invasive bladder cancer, where the disease remains confined to the inner lining of the bladder and may include tumors.
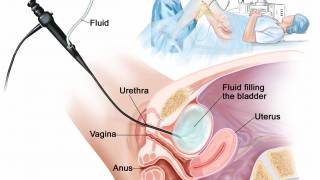
Nogapendekin alfa inbakicept (Anktiva) mixed with BCG is administered via a liquid that is diluted and then delivered into the bladder through a catheter inserted into the urethra.
The BCG (Bacillus Calmette-Guérin) vaccine, which has been deployed for approximately 100 years to reduce tuberculosis, has become a standard immunotherapy for early-stage bladder cancer. It is delivered directly into the bladder to stimulate an immune response.
Anktiva's mechanism of action involves the direct, specific stimulation of CD8+ T cells and Natural Killer cells through beta-gamma T-cell receptor binding, thereby generating memory T cells while avoiding stimulation of T-regulatory cells.
As of July 4, 2025, this medicine was approved through the International Recognition Procedure. The approval was granted to Serum Life Science Europe GmbH.
On May 27, 2025, ImmunityBio announced a collaboration to introduce the Cancer BioShield platform, along with Anktiva, to Saudi Arabia and the broader Middle East.
In the United States, ImmunityBio, Inc.'s BioShield platform, powered by Anktiva, was approved by the U.S. FDA for similar indications in April 2024. It is now commercially available at over 60 cancer centers in the U.S.
On July 1, 2025, ScienceDirect published a systematic review highlighting an array of novel intravesical therapies that demonstrate efficacy in bladder cancer patients.
Our Trust Standards: Medical Advisory Committee