Rotavirus Vaccine Candidate Delivered By Dissolvable Microarray
Emory University and Micron Biomedical recently announced the first clinical trial for a novel rotavirus vaccine, CC24.
This vaccine candidate is delivered using dissolvable microarray technology and is currently enrolling participants in the Phase 1 study, which launched in mid-June 2025.
Notably, this trial represents the first clinical evaluation of a drug or vaccine administered via patch or microarray, sponsored by the U.S. Centers for Disease Control and Prevention (CDC).
"CDC recognizes the potential of this groundbreaking clinical trial, which will test the safety of both our novel vaccine, CC24, in adults and the vaccine's delivery with 'patch' technology," says Dr. Demetre Daskalakis, Director of CDC's National Center for Immunization and Respiratory Diseases, in a press release.
"The trial marks significant progress in vaccine technology innovation and is a critical step toward saving more children from rotavirus illness and death."
Rotavirus infection is a leading cause of diarrheal deaths among children, particularly in low and middle-income countries where existing oral vaccines are often less effective. CC24 is a uniquely inactivated rotavirus vaccine developed by the CDC to provide an alternative to the oral administration of rotavirus vaccines.
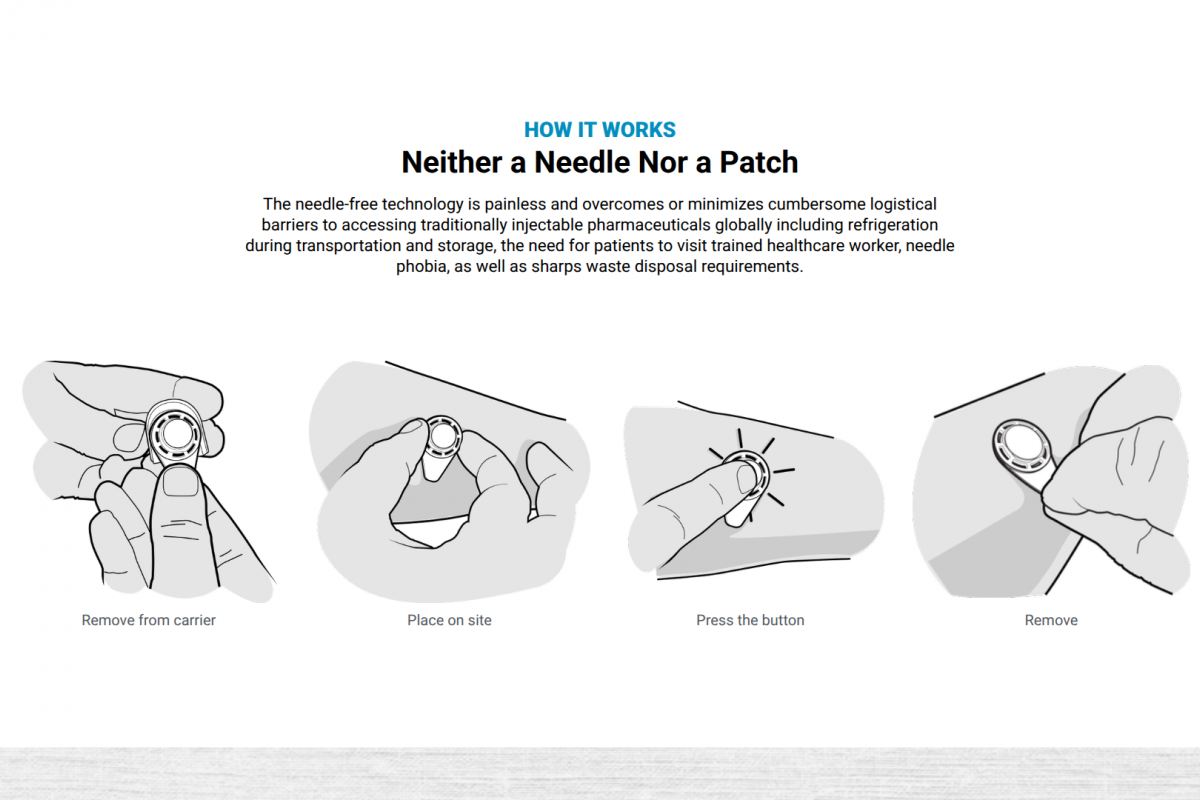
Micron Biomedical's unique needle-free technology rapidly delivers vaccines and therapeutics via dissolvable microarray compounds that are painlessly pressed into the upper layers of the skin.
As of July 2, 2025, U.S.-FDA-approved rotavirus vaccines are available.
Our Trust Standards: Medical Advisory Committee


