Chikungunya Vaccines 2026
Chikungunya Vaccines February 2026
Chikungunya vaccines have been commercially available since 2023 and are approved in various countries, including the United States, as of 2026 Like many vaccines, Chikungunya virus (CHIKV) vaccine technologies, such as live-attenuated virus vaccines, inactivated viral vaccines, recombinant viral vaccines, chimeric alphavirus candidates, DNA vaccines, and virus-like particles, focus on optimizing the balance between efficacy, immunogenicity, and safety, according to the World Health Organization (WHO).
Chikungunya Vaccine Approved and Available in 2026
The U.S. CDC's Advisory Committee on Immunization Practices (ACIP) issued recommendations for the CHIKV vaccine in April 2025, which were adopted by the HHS Secretary on May 13, 2025. As of May 21, 2025, these recommendations are the official guidelines of the CDC.
In the United Kingdom, IXCHIQ® and Vimkunya® vaccines have been reviewed by the Joint Committee on Vaccination and Immunization as of August 7, 2025, and guidance will be drafted for the UK Health Security Agency's green book,' Immunization against infectious disease. Health professionals offering these vaccines must ensure they are adequately informed on their use.
The IXCHIQ chikungunya vaccine from Valneva SE was approved in the U.S. in 2023 and in Canada, Europe, the United Kingdom, and Brazil. On August 22, 2025, it was removed from the U.S. market.
VIMKUNYA® is a virus-like particle vaccine produced by Bavarian Nordic A/S. It was approved in the U.S. and Europe in 2025. It became commercially available at travel clinics and pharmacies in 2026.
Chikungunya Vaccine Candidates 2026
The WHO states that several advanced chikungunya vaccine candidates are either undergoing regulatory review or have already completed it.
Bharat Biotech International Ltd.'s Chikungunya vaccine candidate, BBV87, is an inactivated whole-virion vaccine based on a strain from East, Central, and South African genotypes.
Moderna's mRNA-1944 vaccine candidate encodes a fully human IgG antibody, initially isolated from the B cells of a patient with a prior history of Dengue. A study published in March 2025 concluded that the results from both mouse and rhesus macaque models indicate that the vaccine could be a candidate for clinical use against CHIKV.
CD8+ T cell CHIKV Adaptive Vaccine candidate. Including the ligandome into the vaccine construct will require the selection of eight to twelve peptides from the CHIKV peptide set (ligandome), all of which meet several specific criteria. In efficacy studies, the following will then need to be completed.
The Access to Advanced Health Institute received an $18 million award from the National Institutes of Health to develop a temperature-stable, single-dose vaccine candidate for the chikungunya virus. The vaccine uses an innovative RNA platform technology.
A CHIKV vaccine candidate based on baculovirus displaying the chikungunya E1-E2 envelope confers protection against clinical challenges in mice. C57BL/6 mice were immunized with non-adjuvanted recombinant baculovirus-induced IgG antibodies against E2, with a predominant IgG2c subtype, neutralizing antibodies, and a specific IFN-γ CD8+ T-cell response. A second dose significantly boosted the antibody response.
CHIKV mRNA vaccines study - the results from both mouse and rhesus macaque models indicate that the vaccine could be a candidate for clinical use against CHIKV. Based on the favorable protective effects described in March 2025, 'we consider both mCV-1 and mCV-2 to be potential candidate vaccines for further evaluation in subsequent phase I studies.'
Chikungunya Infections in Infants
This study describes the epidemiological characteristics, clinical presentation, and evolution of unvaccinated patients with CHIKV admitted to the Pediatric Emergency Department in Asunción, Paraguay, during the 2023 epidemic. Results showed a predominance of febrile infants with altered PAT who presented septic shock within the first 24 hours of hospital admission, 15% of patients with seizures, and 2.5% who died.
Chikungunya Virus Mutations
A study published in May 2025 stated the ongoing large outbreak in Sri Lanka is due to the Indian Ocean lineage and the E1:K211E/E2:V264A sublineage of the Chikungunya virus, which has acquired specific, previously uncharacterized mutations. The impact of these mutations on vaccine effectiveness has not been disclosed.
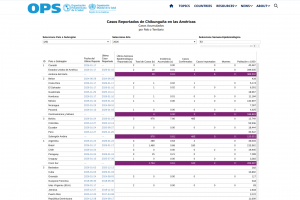
Chikungunya Outbreaks
As of 2025, millions of people live in areas where Chikungunya is endemic. Chikungunya outbreak news is posted at this link.