Children May Need Full-Dose Yellow Fever Vaccination

Each year, millions of children travel to countries where the mosquito-borne Yellow fever virus is present. Due to inconsistent access to effective vaccines, various health agencies have recommended altering the standard vaccination schedule for all travelers.
A recent study published in The Lancet found that a low-dose (500 IU) Yellow fever vaccine does not provide the same level of protection for infants as the standard dose.
The study's findings highlight significant differences in immune responses between children and adults.
Published on January 13, 2026, this randomized, double-blind, phase 4 clinical trial enrolled infants aged 9 to 12 months who had not received a prior yellow fever vaccination. Participants received either a standard dose (over 13,000 IU) or a 500 IU dose of the Institut Pasteur de Dakar 17D-204 yellow fever vaccine.
The primary outcome was seroconversion at 28 days post-vaccination, defined as a four-fold or greater increase in antibody titres.
Results showed 99% seroconversion (95% CI: 96–100%; 177 of 179 infants) for the Standard dose. And the 500 IU dose: 93% seroconversion (95% CI: 88–96%; 166 of 179 infants).
The difference was -6.15 percentage points (95% CI: -10.27 to -2.02).
Both groups had comparable safety profiles, with 12 serious adverse events reported (8 in the low-dose group and 4 in the standard group), all unrelated to the vaccine.
These researchers wrote that the findings indicate that the evidence for fractional dosing in adults does not apply to infants, who may need higher doses for adequate protection.
And while a lower seroconversion rate with the 500 IU dose might be tolerable during outbreaks, it's not advisable for routine vaccination programs.
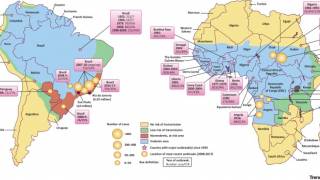
In a related commentary also published by The Lancet, Lance Turtle, MBBS, PhD, of the University of Liverpool, said during yellow fever outbreaks, the vaccine has been administered in fractional doses, typically a fifth of a standard dose.
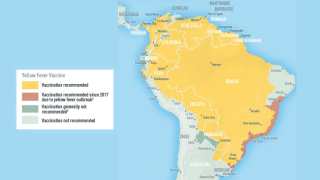
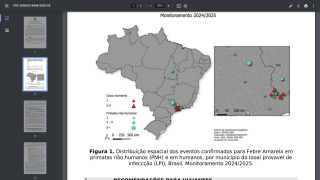
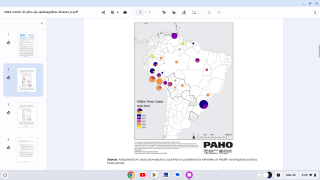
According to Don Hackett, publisher of Vax-Before-Travel, this study reinforces the need to use standard full doses in infants for adequate long-term protection against yellow fever when visiting endemic areas in countries such as Brazil.
As of 2026, the U.S. CDC recommends the YF-VAX yellow fever vaccine for children 9 months and older traveling to or living in high-risk areas or where vaccination is required for entry. However, infants under 9 months, pregnant women, and those with certain conditions should avoid vaccination.
When departing from the United States for at-risk areas in 2026, travel vaccine experts can offer their advice regarding the U.S. FDA-approved YF-VAX vaccine.
Our Trust Standards: Medical Advisory Committee