Can Fractional Yellow Fever Vaccinations Decrease Deaths in the Americas
While the Yellow fever vaccine has been found highly effective when administered as a single dose, researchers have continued to examine the minimum dose requirements for seroconversion.
These researchers say the potential advantages of offering fractional doses are increased vaccinations and reduced under-vaccination, which have become a measurable health concern in the Region of the Americas over the past few years.
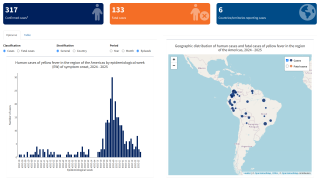
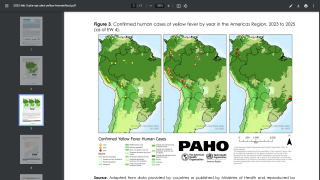
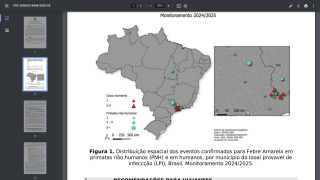
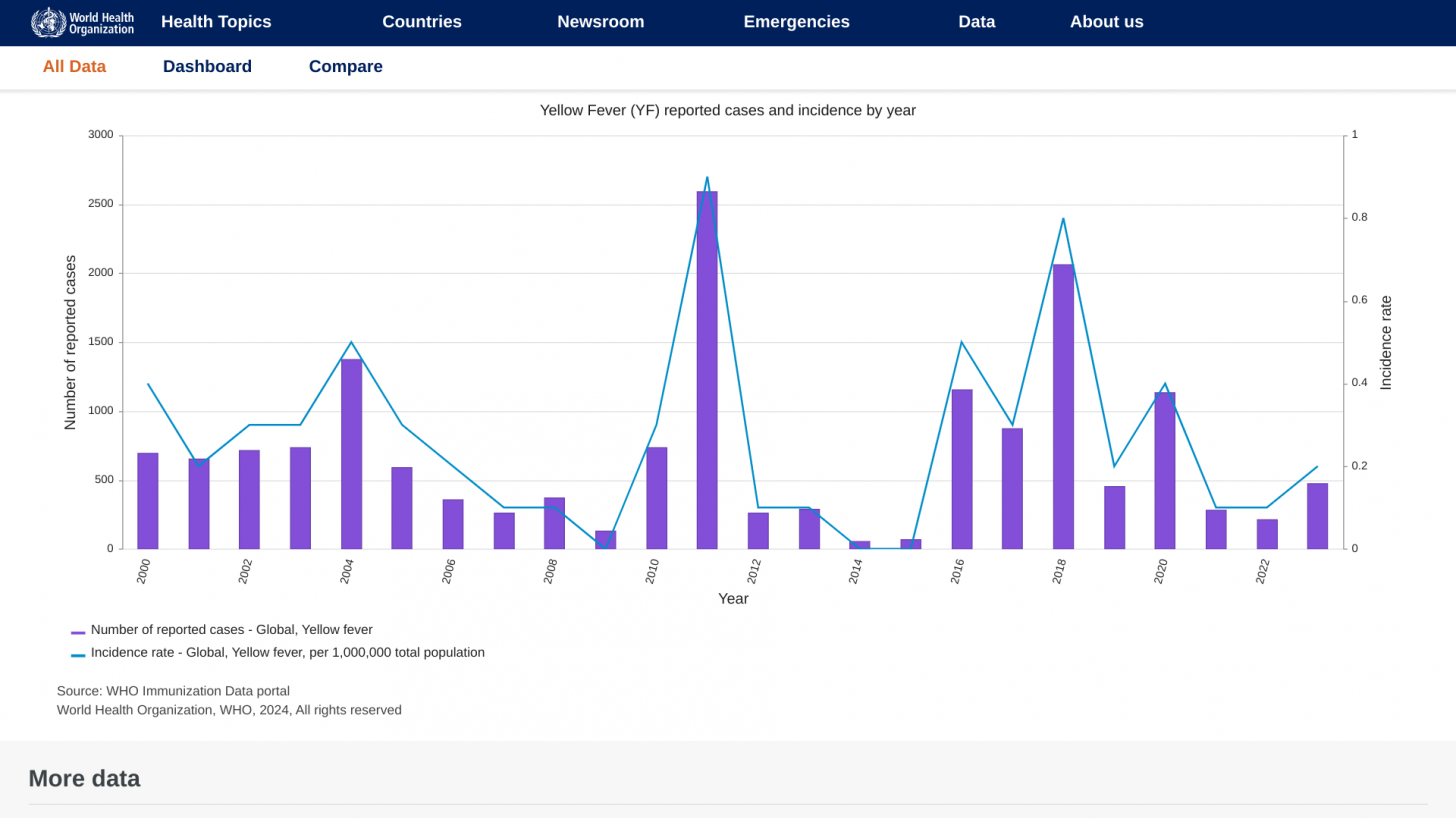
For example, 61 human cases of yellow fever were confirmed in the Region of the Americas in 2024, of which 30 were fatal (case fatality ratio, 50%).
According to a new study, fractional dosing can protect people similarly to a full dose.
On February 19, 2025, the NEJM published an Orginal Article that concluded that Institut Pasteur de Dakar 17D-204 yellow fever vaccination fractional dose of 500 IU was noninferior to the standard dose of 13,803 IU for producing seroconversion within 28 days.
Noninferiority was shown if the lower boundary of the 95% confidence interval for the difference in the incidence of seroconversion between the fractional dose and the standard dose was higher than -10 percentage points.
Previously, The Lancet Infectious Diseases published an Opinion on April 28, 2023, saying fractional dose yellow vaccination in an emergency or reactive mass vaccination campaign has become essential in stretching the limited vaccine stockpile over a much larger population.
The Lancet also published results from a clinical study in January 2021 (updated Feb. 2022), concluding, 'Fractional doses of all WHO-prequalified yellow fever vaccines were non-inferior to the standard dose in inducing seroconversion 28 days after vaccination, with no major safety concerns. These results support using fractional dosage in the general adult population for outbreak response in a vaccine shortage.'
Moreover, the WHO's initial position on fractional yellow fever vaccine was set out in the 2013 position paper.
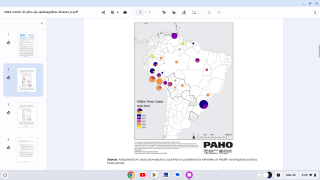
According to the Pan American Health Organization (PAHO) rapid risk assessment on February 14, 2025, yellow fever under vaccinations remains a public health concern.
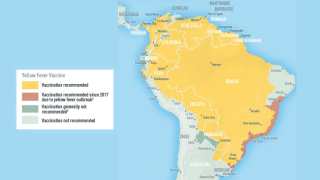
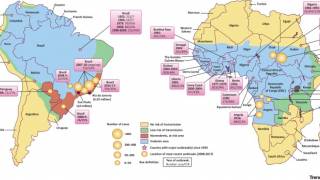
Between 2014 and 2023, yellow fever vaccine coverage declined in about half of the countries with yellow fever endemic areas in the Americas. Six countries had less than 80% coverage: Argentina, Bolivia, Brazil, Panama, Peru, and Venezuela.
This under-vaccination rate is associated with the increase of yellow fever cases in late 2024 and the beginning of 2025 in endemic countries of the Americans, such as Brazil and Columbia.
PAHO says the overall risk of this event in the Americas Region, especially in endemic countries, is classified as ¨High¨ with a ¨High¨ level of confidence based on the available information.
Regardless of the vaccine's dosage, the PAHO and the U.S. Centers for Disease Control and Prevention recommend international travelers be fully protected against the mosquito-transmitted Yellow fever virus before visiting endemic areas in 2025.
In the United States, travel clinics and pharmacies offer the U.S. FDA-approved yellow fever vaccine YF-VAX.
Our Trust Standards: Medical Advisory Committee