Dengue-naïve People Frequently Report Vaccine-related Reactions
A research study was recently conducted to assess the safety and tolerability of the tetravalent live-attenuated dengue vaccine, which is commercially known as Qdenga®. The study provides important insights into reactogenicity and may help improve vaccination strategies in dengue-naïve populations.
The study results were published in the Journal of Travel Medicine on February 2, 2025. Vaccine-related reactions were frequently reported, predominantly after the first dose in dengue-naïve participants.
While vaccine coadministration was a common strategy, it did not significantly increase side effects.
After the first dose, 51% of the participants reported systemic reactions, such as headache (40% (190/474)), weakness (40% (189/474)), and malaise (32% (154/474)), which were most pronounced between days 7 and 11 after vaccination.
After the second dose, localized signs and symptoms such as pain at the injection site (22% (n = 55/250)) were more common. Fever was more common after the first dose (20% (96/474)) vs. 2% (6/250) after the second.
A total of 334 (28%) coadministrations were reported, whereby AEs were reported in 47% (157/333) of participants, with the highest prevalence observed when combined with the Japanese encephalitis vaccine (56.8%, (42/74)).
Differences in age groups were observed, with decreased reactions in older people (≥ 65 years).
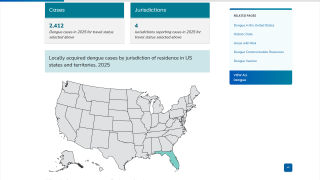
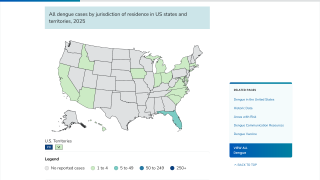
As of February 2025, Qdenga is not offered in the United States and is in limited supply globally.
Later this year, Butantan Institute's single-dose, tetravalent, live attenuated Butantan-DV dengue vaccine may become available in Brazil, where it conducts phase 3 clinical trials.
Our Trust Standards: Medical Advisory Committee