During 2024, the United States reported several measles outbreaks primarily related to unvaccinated international travelers. According to new reports, the State of Texas may lead this unfortunate list in 2025.
Today, the Texas Department of State Health Services (DSHS) announced two confirmed measles cases in Gaines County residents, located southwest of Lubbock, Texas. Both instances involve unvaccinated school-age children who were hospitalized in Lubbock.
As of January 30, 2025, these children have been discharged.
These newly identified cases are in addition to two confirmed measles cases reported in Harris County in 2025.
The Houston Health Department (HHD) identified two confirmed measles cases associated with international travel. Both adults were unvaccinated against measles.
HDD says anyone exposed to measles should monitor themselves for symptoms, including a rash, high fever, cough, runny nose, and red, watery eyes. Symptoms can appear 7 to 21 days after exposure. If you show symptoms of measles, call your healthcare provider to make arrangements for evaluation and treatment.
On January 23, 2025, HHD stated, 'Due to the highly contagious nature of this disease, additional (measles) cases may occur.'
Houston and Harris County are home to about 5 million people and are gateway cities with two international airports.
Crockett Tidwell RPh, CDCES, CTH, informed Vax-Before-Travel News, "Measles is extremely contagious; nine9 out 10 people in the same room will become infected if they do not have immunity."
"All it takes is one international traveler to infect every vulnerable person they come in contact with when they come home," added Tidwell, Clinical Services Manager, International Society of Travel Medicine Certificate in Travel Health™.
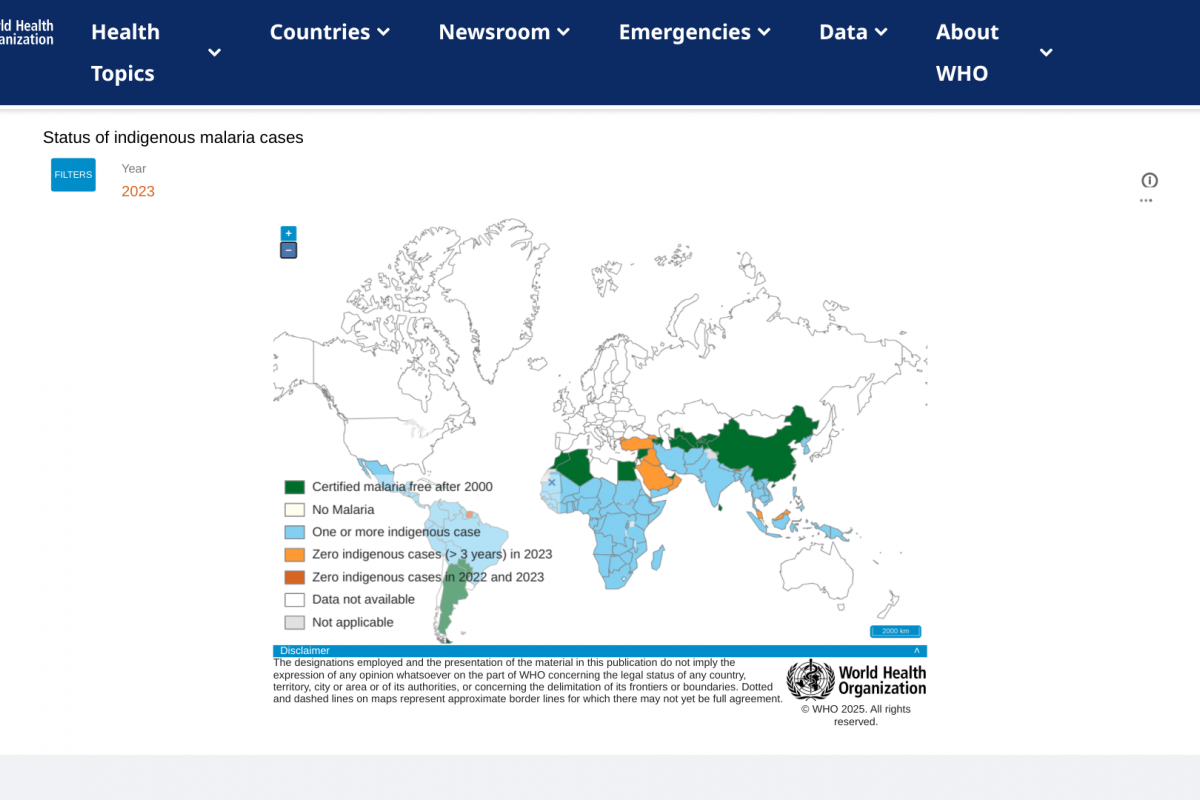
To alert travelers of the global measles risk, the U.S. CDC recently updated a Travel Health Advisory, which identified 59 countries reported measles cases. The CDC recommends people speak with a travel vaccine expert about immunization options before visiting these countries in 2025.