Mpox Vaccines
Mpox Vaccines June 2025
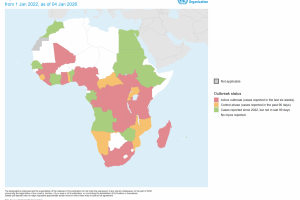
The World Health Organization (WHO) announced in June 2025 that the mpox upsurge continues to meet the WHO criteria of a public health emergency of international concern. The Strategic Advisory Group of Experts on Immunization, along with other health agencies, has recommended vaccination against mpox disease and publishes the Emergency Use Listing of mpox vaccines. As of June 10, 2025, four vaccines are in use to protect people against mpox. According to the WHO, travelers who may be at risk, as assessed by their healthcare provider, may wish to consider mpox vaccination. However, mass vaccination is not recommended.
The United States Food and Drug Administration (FDA) Approved the JYNNEOS® (MVA-BN, IMVANEX®, IMVAMUNE®) Smallpox and Mpox Vaccine on September 24, 2019. The U.S. began offering Bavarian Nordic's JYNNEOS to healthcare staff in Boston on May 24, 2022, and then to consumers throughout the U.S.
Japan's K.M. Biologics' LC16 "KMB" freeze-dried smallpox vaccine has been approved by Japan's Ministry of Health, Labor, and Welfare, as well as by other countries, for the prevention of mpox disease. A review published by John D. Grabenstein and Adam Hacker summarizes the human and pivotal animal data establishing the safety and efficacy of LC16m8.
The ACAM2000 live vaccinia virus vaccine is authorized to prevent mpox and smallpox infections in various countries, including the United States. The saf ty profile of the ACAM2000 vaccination includes side effects and risks.
The State Research Center of Virology and Biotechnology Vector Laboratories produces the OrthopoxVac vaccine.
Mpox Vaccine Candidates
The Sinopharm mpox vaccine candidate clinical trial was approved in China in September 2024. The Bei ing Institute of Biological Products Co., Ltd. (BIBP), also known as Sinopharm, confirmed that China's drug authority has granted permission to launch an mpox vaccine based on replication-defective mpox viruses that cannot spread but can confer immunity to a person. China classified mpox as a Class B infectious disease in 2022.
Moderna Inc.'s mpox vaccine candidate mRNA-1769 prevented severe disease and reduced virus levels in monkeys. The results of a preclinical study were published in Cell on September 4, 2024. A study published in December 2024 concluded, 'A single vaccination provided considerable protection, enhanced by boosting, for at least 4 months. Protective immunity was related to the amount of mRNA inoculated, which correlated with neutralizing antibody levels. Furthermore, immunocompetent and immunodeficient mice lacking mature B and T cells that received serum from mRNA-immunized macaques before or after the VACV challenge were protected.'
Tonix Pharmaceuticals Holding Corp. confirmed the development of X-801 as a vaccine candidate targeting mpox. As of September 2024, Tonix has completed non-human primate studies showing protection from the Clade 1 mpox virus. Seth Leerman, M.D., Chief Executive Officer of Tonix, stated, "In animal studies, TNX-801 has shown single-dose protection against a lethal challenge of Clade I monkeypox virus administered by the intratracheal route." TNX-801 is an attenuated live-virus vaccine based on synthesized horsepox that has been shown to provide single-dose immune protection against a monkeypox challenge with better tolerability than 20th-century vaccinia live-virus vaccines in animals. On August 26, 2024, Tonix partnered with Bilthoven Biologicals on vaccine development.
The Institute of India, Reliance Life Sciences, and Dr. Reddy's Laboratories are among the firms collaborating to produce a vaccine for the MPXV in India. The Indian Council of Medical Research (ICMR) initiated this in collaboration on July 27, 2022, for the development of in-vitro diagnostic kits and vaccine candidates against the MPox virus.
Emergex Vaccines Holding Limited announced on October 18, 2022, that it has formulated and confirmed the synthesis and assembly of a CD8+ T cell Adaptive Vaccine for smallpox and mpox, comprised predominantly of early "eclipse phase" antigens.
EpiVax, Inc.'s "Epitope-Driven Vaccine" is a smallpox vaccine candidate predicted to be highly effective against mpox. The development of VennVax, a DNA-prime, peptide-boost multi-T-cell epitope poxvirus vaccine, was funded by the U.S. National Institutes of Health (SBIR grant #R43AI058376).
The Hopoxvac vaccine, developed by the Vektor laboratory, was registered in 2022.
A study published on March 26, 2025, found that co-immunization with Mix-12 and MPX-EPs provides complete protection against MPXV challenge. Overall, these results suggest a practical approach to enhancing the immune protection of mRNA vaccines through the specific coordination of humoral and cellular immune responses.
Mpox Treatment
In June 2025, this living guideline from WHO incorporates new evidence to dynamically update recommendations for clinical management and IPC for mpox infection. The Africa CDC announced in January 2025 that brincidofovir, an antiviral treatment for mpox infection from Emergent BioSolutions, had launched a clinical trial.
Mpox Virus Disease
Monkeypox is a zoonotic virus that is transmitted from one person to another through physical contact with lesions, body fluids, respiratory droplets, and contaminated materials. The virus causes monkeypox disease, a member of the Orthopoxvirus genus in the family Poxviridae. According to the WHO, the monkeypox virus has two distinct genetic clades: the Congo Basin and the West African clade.