Mpox Protective Antibodies Quickly Wane After Jynneos Vaccination or Infection
A recent retrospective study published in the Journal of Infectious Diseases provides insights into the long-term persistence of immunological memory against the monkeypox virus (MPXV).
Published on January 5, 2026, this new study demonstrates that MPXV-specific neutralizing antibodies (NAbs) often decline to undetectable levels more than two years after either natural mpox infection or vaccination with the third-generation JYNNEOS® (MVA-BN®) vaccine.
Conducted in Italy, the study included 90 adult men, of whom 48 had a history of laboratory-confirmed mpox infection, and 42 had received the standard two-dose JYNNEOS vaccine series.
At more than two years post-infection or vaccination, only 24.4% (22 out of 90) had detectable positive NAbs.
Using a more sensitive criterion, 58.8% (53 out of 90) remained positive.
Furthermore, individuals with prior mpox infection were more likely to retain detectable NAbs at the IC50 level (68.75% compared to 47.62% in vaccinees; p=0.042).
These researchers wrote that higher NAb titers measured at six months strongly predicted better persistence beyond two years (univariate odds ratio = 6.45; p=0.003).
In multivariable analysis, a history of childhood smallpox vaccination was the strongest independent factor associated with higher NAbs after more than 2 years (odds ratio = 5.37; p = 0.020).
Prior mpox infection was associated with a marginal increase in odds (odds ratio = 2.08; p = 0.073).
The study authors conclude that MPXV-specific NAbs "waned at greater than two years from previous infection or vaccination, often becoming undetectable."
These findings align with recent studies indicating a faster decline in vaccine-induced antibodies than in those following natural infection and highlight the potential role of historical smallpox vaccination in enhancing long-term immunity against orthopoxviruses.
While antibody levels decline, the clinical significance of this waning immunity remains unclear. Real-world data suggest that MVA-BN vaccination continues to provide substantial protection against severe disease, possibly due to T-cell memory or other immune mechanisms not measured in this study.
As of January 7, 2026, various health authorities advise travelers to regions with ongoing mpox transmission to consult healthcare providers about vaccination options, as pre-travel immunization remains a crucial preventive measure.
However, the U.S. CDC wrote in late 2025 that boosters (third) doses are not currently recommended, except for certain people who work with orthopoxviruses in research or monkeypox diagnostic laboratories.
Other countries, such as France, offer different booster dose advice.
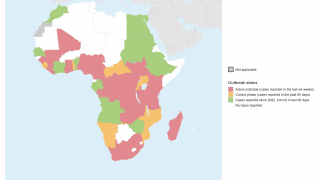
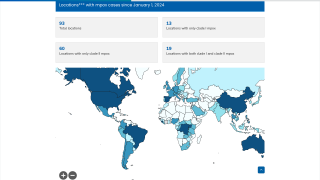
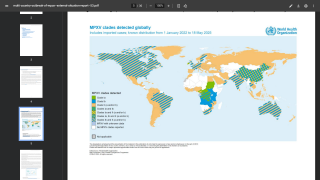
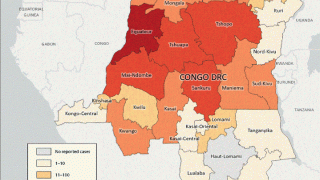
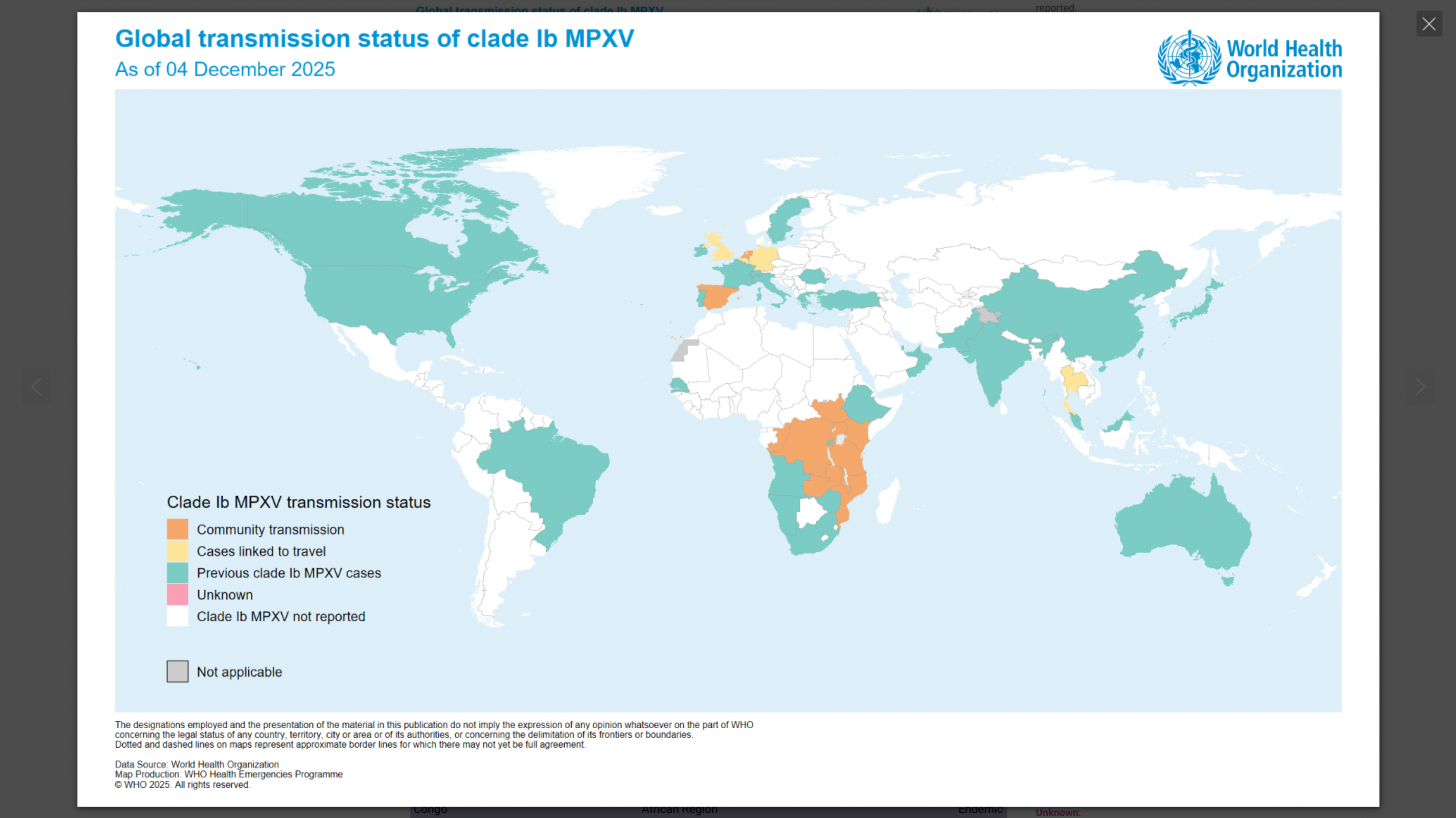
This World Health Organization report (December 2025) provides an overview of the mpox epidemiological situation, based on global surveillance data reported since January 2022. Distinct mpox clades and subclades are affecting diverse populations across different geographical regions, each exhibiting distinct transmission dynamics.
Institutional resources funded this mpox study; not the vaccine manufacturer, no conflicts reported.
Our Trust Standards: Medical Advisory Committee