Should Mpox Vaccination Be Offered to More Children and Adolescents

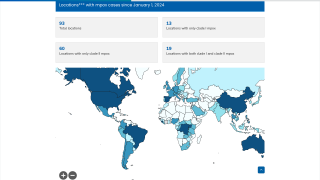
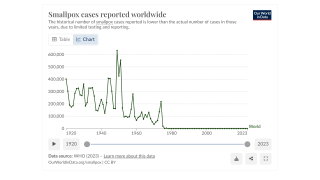
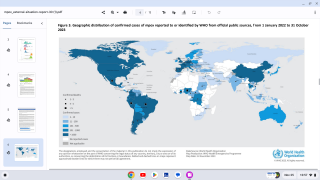
Since May 2022, a global outbreak of clade IIb human monkeypox virus (MPXV) has caused more than 100,000 confirmed infections in various countries.
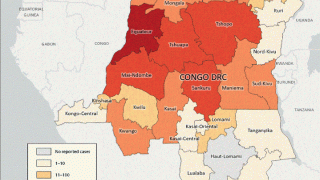
According to the World Health Organization (WHO) 53rd External Situation Report published on May 29, 2025, ten countries in the African Region have reported community transmission of mpox due to clade Ib MPXV in the past six weeks.
However, within the United States, neither mpox clade has been common in 2025.
To reduce these sexually transmitted MPXV infections, the U.S. Food and Drug Administration, the WHO, and other health agencies have issued authorizations for Bavarian Nordic's JYNNEOS® (MVA-BN), a third-generation, live-attenuated vaccine derived from a modified vaccinia Ankara virus lineage.
More than 720,000 JYNNEOS doses have been administered in seven African countries.
According to a research article published by The Lancet Infectious Diseases on May 21, 2025, observational studies conducted over the past few years have demonstrated that JYNNEOS is effective in preventing mpox.
Compared with unvaccinated people, individuals who got mpox after pre-exposure vaccination with JYNNEOS had a 59% lower risk of having disseminated lesions across multiple anatomical areas.
Furthermore, vaccine effectiveness against progression to disseminated lesions was similar regardless of whether individuals received one or two pre-exposure doses.
These researchers estimated vaccine effectiveness against progression to disseminated lesions to be 67% among people who were HIV negative and 45% among people who were HIV positive.
However, protection was apparent only among people who were HIV positive with high CD4 count (≥350 cells per μL).
They also found that pre-exposure vaccination reduced the likelihood of people being admitted to the hospital or experiencing symptoms such as fever, chills, and lymphadenopathy.
Finally, they found that post-exposure prophylaxis with JYNNEOS provided 16% effectiveness in preventing progression to disseminated lesions.
Such studies have also reported a lower risk of hospitalization among vaccinated individuals compared with those who are unvaccinated.
These researchers wrote, 'Understanding of the relationship between vaccination and mpox clinical presentation or progression can inform awareness of the need for testing among people with less-severe manifestations, who are at risk of transmitting infection.'
As of May 29, 2025, the U.S. CDC's Advisory Committee on Immunization Practices recommends vaccination for certain men who were diagnosed with any sexually transmitted infection or had multiple sexual partners within six months; people who have had sex in a commercial venue or significant public event within six months; people who anticipate having any of these exposures; and sexual partners of individuals with these risk factors.
The CDC says 'Vaccination is an important tool in stopping the spread of mpox.'
'If you have certain risk factors that make you eligible, you can help protect yourself from mpox by getting the mpox vaccine. Contact your healthcare provider, local pharmacy, or local health department to find the JYNNEOS vaccine in your area.'
A recent CDC modeling result published on May 11, 2025, indicates that Mpox infection can be severe in children. Very few children in the U.S. have acquired immunity from either previous infection with the virus that causes mpox or vaccination with mpox or smallpox vaccines.
The CDC says both types of MPXV (clade I and clade II) can be prevented, reducing the risk of children living with infected adults.
Exposure to a household contact with mpox is the predominant route of exposure for children, while sexual contact is the predominant route of exposure for adolescents.
Children and adolescents with close contact to people with suspected, probable, or confirmed mpox may be eligible for post-exposure prophylaxis through vaccination, immune globulin, or antiviral medication.
Currently, Mpox vaccines are offered at health clinics and pharmacies in the U.S.
Our Trust Standards: Medical Advisory Committee