$143 Million Expands U.S. Mpox Smallpox Vaccine Stockpile
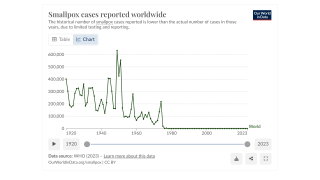
In 1967, the World Health Organization (WHO) embarked on an ambitious campaign to eradicate smallpox. Over several years, an immunization strategy was executed, culminating in the last natural case of smallpox in 1977.
In 1980, the WHO triumphantly declared smallpox eradicated, a historical milestone celebrating modern vaccine production and solidifying smallpox as the only infectious disease to be eliminated.
This success stands as a testament to the power of global vaccination programs.
To ensure the United States is well prepared to combat a smallpox outbreak, it has secured stockpiles of preventive vaccines. Effective today, the U.S. government is expanding its inventory with a freeze-dried formulation of the JYNNEOS® (MVA-BN®, IMVAMUNE®) vaccine.
The liquid-frozen formulation, approved by the U.S. FDA in March 2025, provides certain advantages over the liquid-frozen formulation in terms of transportation, storage conditions, and shelf life, all essential for long-term stockpiling.
Bavarian Nordic A/S today announced that the Biomedical Advanced Research and Development Authority (BARDA) has exercised additional options valued at $143.6 million under an existing contract.
The options support the manufacturing and supply of freeze-dried JYNNEOS by converting bulk vaccine previously manufactured under other contract options.
In a press release on May 6, 2025, Paul Chaplin, President & CEO of Bavarian Nordic, commented, "Following the recent FDA approval of the freeze-dried formulation of our smallpox/mpox vaccine, we applaud the U.S. government's steadfast commitment to improving national health security through the exercise of these options."
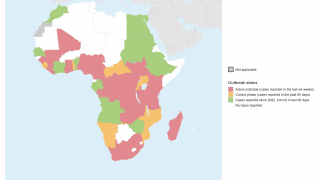
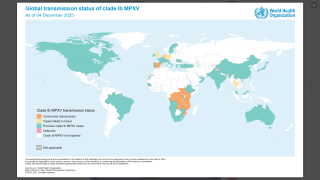
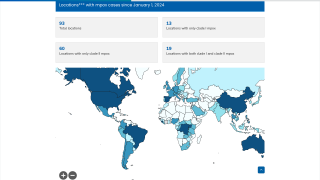
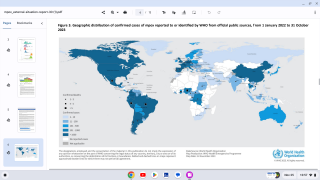
Approved by the FDA in 2019, JYNNEOS was the first smallpox vaccine successfully developed under Project BioShield. The Company has supplied a liquid-frozen formulation of JYNNEOS to the U.S. government for stockpiling since 2010 and in deployed response to the clade 2 mpox global outbreak that began in May 2022.
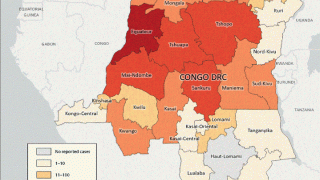
The first case of clade I mpox in the U.S. was detected in November 2024, against which JYNNEOS is also used. As of early May 2025, nearly 1 million doses have been deployed in Africa.
From a vaccine efficacy perspective, recent studies have confirmed JYNNEOS is highly effective against mpox.
BARDA has supported the development of a freeze-dried formulation of the vaccine to replenish the stockpile and, in 2017, awarded the Company a ten-year contract, which includes options valued at $299 million for the fill and finish of freeze-dried vaccines, of which $284 million has been exercised to date.
This project has been supported in whole or in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; BARDA, under contract number HHSO100201700019C.
As of May 2025, JYNNEOS remains commercially available in the U.S. for the mpox indication, but not for smallpox.
Our Trust Standards: Medical Advisory Committee