Just One HPV Vaccine Dose Benefit Similar to Two

A recent Original Article indicates that a single dose of the FDA-approved human papillomavirus (HPV) vaccine may be as effective as two doses in protecting young women in low-income, hard-to-reach countries.
Published in The New England Journal of Medicine on December 3, 2025, this study (NCT03180034) included 20,330 participants who were enrolled and underwent randomization, and 3005 unvaccinated participants were enrolled in the survey.
The noninferiority analysis showed that one dose of vaccine was noninferior to two doses in preventing HPV16 or HPV18 infection, which causes more than 77% of cervical cancers worldwide.
The rate difference between one and two doses of the bivalent vaccine was −0.13 infections per 100 participants (95% confidence interval [CI], −0.45 to 0.15; P<0.001 for noninferiority), and the difference between one and two doses of the nonavalent vaccine was 0.21 infections per 100 participants (95% CI, −0.09 to 0.51; P<0.001 for noninferiority).
The vaccine effectiveness was at least 97% in each of the four trial groups. And no safety concerns were identified.
These researchers concluded that one dose of either a bivalent or nonavalent HPV vaccine provided protection against HPV16 or HPV18 infection and was not inferior to two doses.
The National Cancer Institute and others funded this study.
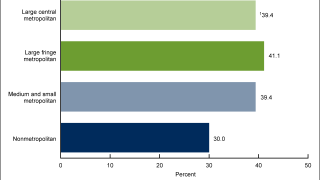
Currently, the U.S. CDC recommends routine vaccination of preteens at age 11 or 12. The CDC's vaccination series recommendations identify two or three doses.
Our Trust Standards: Medical Advisory Committee