Cervical Cancer Home Testing Guidance Announced

The Health Resources and Services Administration (HRSA), a federal agency under the U.S. Department of Health and Human Services, today announced a groundbreaking update to its Women's Preventive Services Guidelines for cervical cancer screening.
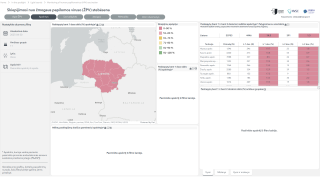
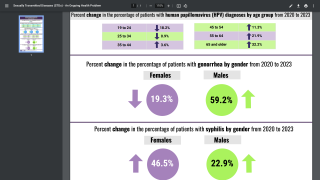
For the first time, the HRSA guidelines include an option for women to self-collect vaginal samples for high-risk human papillomavirus (hrHPV) testing, either privately in a health care provider's office or at home using FDA-approved kits.
As of January 5, 2026, this marks the first federal health agency guidance to explicitly endorse self-collection as part of routine screening, aiming to increase accessibility and reduce barriers to this critical preventive service.
This policy change aims to address common barriers to screening, including discomfort with pelvic exams, access challenges in rural areas, and scheduling difficulties, potentially increasing participation rates and saving lives.
The updated recommendations were detailed in a publication released today in the Journal of the American Medical Association.
These authors stated, 'Cervical cancer screening is highly effective at detecting precancerous and early-stage cancers for women who undergo recommended screening.'
'Self-collection for hrHPV screening is an important and innovative breakthrough in the fight against cervical cancer and has the potential to increase screening rates and save lives.'
'By reducing testing barriers, expanding choice, empowering women, and eliminating patient cost sharing, HRSA's new Women's Preventive Services guidelines for cervical cancer screening are a powerful step forward for women's health across the United States.'
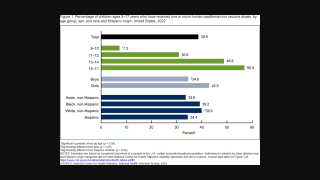
As a cancer prevention option, HPV vaccines are offered at clinics and pharmacies in the U.S.
Our Trust Standards: Medical Advisory Committee