HPV9 Vaccines Coming to Latin America

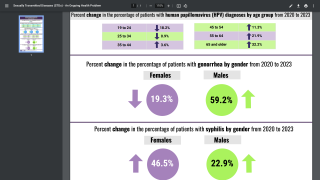
Over the past decade, human papillomavirus (HPV) vaccinations have prevented thousands of cervical cancer cases worldwide.
Beginning in July 2025, the enhanced 9-valent HPV vaccine will become more available in the Region of the Americas.
The Pan American Health Organization (PAHO) Revolving Fund announced on February 7, 2025, that the general availability of the 9-valent HPV vaccine will be easier and more affordable for Latin American countries.
This PAHO initiative is expected to help immunization programs access tools to protect their populations against the most prevalent genotypes in cervical cancer and reduce the overall burden of HPV-related diseases. The HPV9 vaccine has five more valencies than its previous version (quadrivalent) against the genotypes.
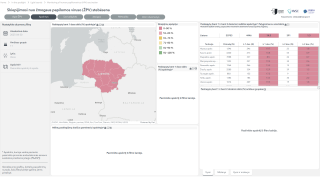
This is an essential PAHO program since, in 2022, more than 78,000 women were diagnosed with cervical cancer, and about 40,000 died from this preventable disease in the Americas.
Furthermore, mortality rates are three times higher in Latin America and the Caribbean as compared to North America, Canada, and the United States.
In a press release, Santiago Cornejo, Executive Manager of PAHO's Regional Revolving Funds, commented, "By working together, we can make life-saving vaccines more accessible to all and continue the goal of eliminating cervical cancer by 2030."
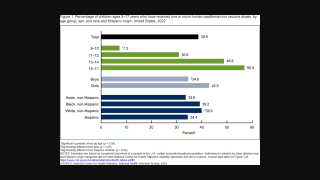
Currently, the HPV9 vaccine is recommended for the entire population from 9 to 26 years of age, depending on the immunization schedules of each country.
The PAHO Elimination Initiative seeks to end more than 30 diseases by 2030, including cervical cancer, using vaccination as a key tool. The goal is to vaccinate 90% of girls before the age of 15 with at least one dose of the HPV vaccine.
The PAHO Revolving Fund allows countries to combine their purchasing power to negotiate better prices and ensure a permanent and timely supply of their vaccines and other related inputs. This approach reduces costs and simplifies the procurement process for participating countries.
WHO prequalifies all vaccines included in the PAHO Revolving Fund portfolio or a reference authority. It seeks to offer quality, safe health technologies that meet technical criteria.
In the United States, Merck's GARDASIL 9® vaccine is the leading HPV vaccine. In 2025, it is generally available for boys and girls at health clinics and pharmacies.
Our Trust Standards: Medical Advisory Committee