Spain, a favorite destination for international travelers in 2026, is currently facing a concerning rise in mpox (monkeypox) cases within the European Union.
Health authorities have reported an increase in infections primarily linked to the more transmissible clade Ib variant of the monkeypox virus (MPXV).
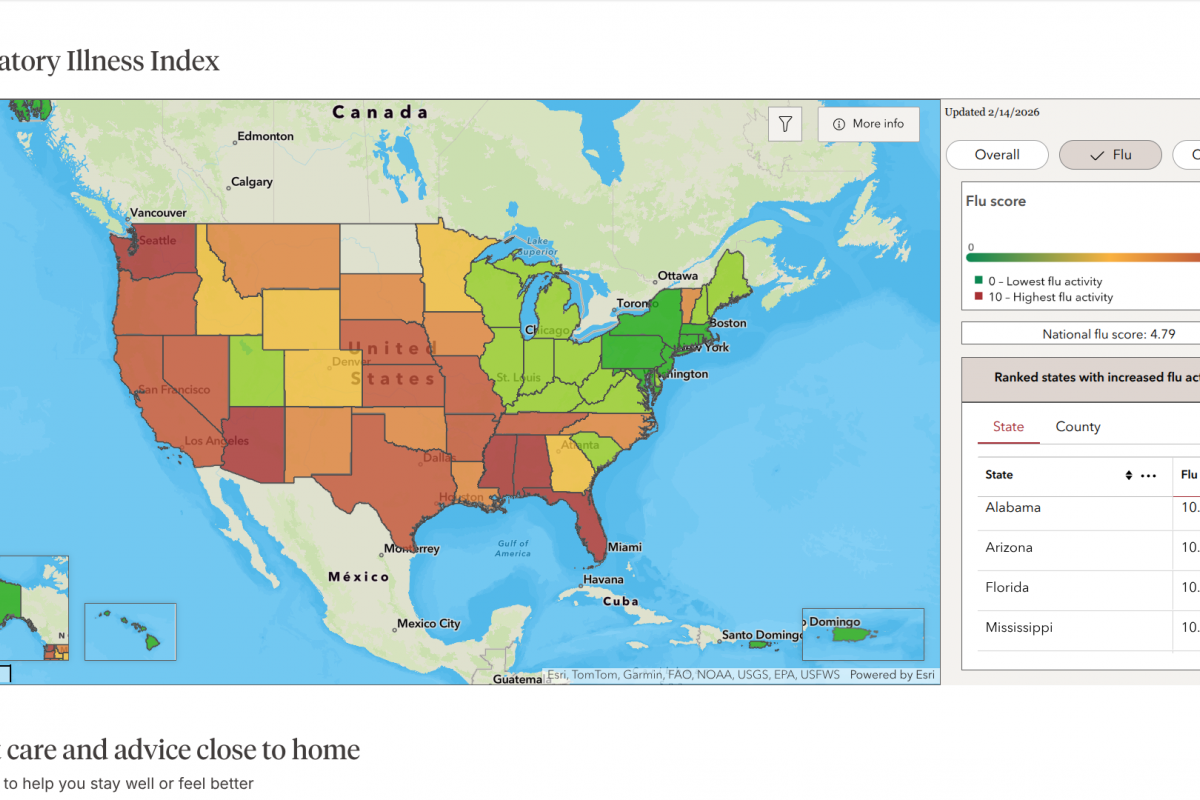
According to the latest update from the European Centre for Disease Prevention and Control (ECDC) on February 18, 2026, Spain has confirmed 84 mpox cases in 2026.
Most of Spain's cases (36 out of 84) involve the MPXV clade Ib strain, which is known for its higher transmissibility compared to the clade II variant that was responsible for the global outbreak in 2022.
This ECDC data for week #8 makes Spain the hardest-hit country in the EU/EEA region, with a total of 255 cases reported across 10 countries during the same timeframe.
MPXV cases are distributed across multiple regions, indicating widespread community transmission rather than isolated clusters.
Specific city-level breakdowns in Spain for 2026 remain limited in public reports. Still, patterns point to urban centers, such as Madrid, which has been a primary hotspot, consistent with historical trends and the location of the initial autochthonous clade Ib case identified in October 2025.
Other regions with likely involvement include Catalonia (Barcelona) and Andalusia (Seville and Malaga), which have seen higher incidences in past mpox surges.
This rise in cases follows sporadic local transmissions reported in late 2025, including the first documented human-to-human transmission of clade Ib outside of Africa in December 2025.
The multi-regional spread underscores the need for nationwide vigilance, as transmissions occur mainly within sexual networks, particularly among men who have sex with men (MSM).
Local data highlights significant gaps in mpox protection. Among 72 mpox cases in MSM, the ECDC reports 66% were unvaccinated.
This low uptake reflects challenges in pre-exposure prophylaxis campaigns.
The MVA-BN (JYNNEOS) vaccine has proven effective in reducing risks, but waning immunity and incomplete coverage are contributing to the current resurgence.
As of late February 2026, the Spanish Ministry of Health, in coordination with the ECDC, has intensified efforts, including enhanced vaccination drives, contact tracing, and community engagement focused on MSM networks.
While the U.S. CDC has included Spain in Measles and Polio Travel Health Notices, it does not mention Mpox as a current risk for visitors.