The University of Oxford announced today that it has launched the world's first Phase II clinical trial of a Nipah virus vaccine candidate in Bangladesh in partnership with the International Centre for Diarrhoeal Disease Research.
This study is essential, as a vaccine is urgently needed, as the disease can be fatal in up to 75% of cases.
Funded by the Coalition for Epidemic Preparedness Innovations, this study will assess the safety and immune response of the ChAdOx1 NipahB vaccine in a region where the virus causes recurrent outbreaks.
The ChAdOx NipahB vaccine was manufactured for this clinical trial by the Serum Institute of India Pvt. Ltd., the world's largest vaccine manufacturer.
Professor Brian Angus, Professor of Medical Practice at the Nuffield Department of Medicine, University of Oxford and Chief Investigator of the trial at the Oxford Vaccine Group, commented in a press release on December 9, 2025, "Starting a Phase II trial in a country affected by regular Nipah outbreaks is a critical step in making sure this vaccine is both effective and relevant to the people who need it most."
"It's an essential part of ensuring equitable access to protection against emerging infectious diseases."
Nipah virus is a deadly disease from the same viral family as measles, the paramyxoviruses.
The World Health Organization recognises it as a research priority due to its pandemic potential. Of the 750 cases recorded since 1998, there have been 415 deaths related to this virus.
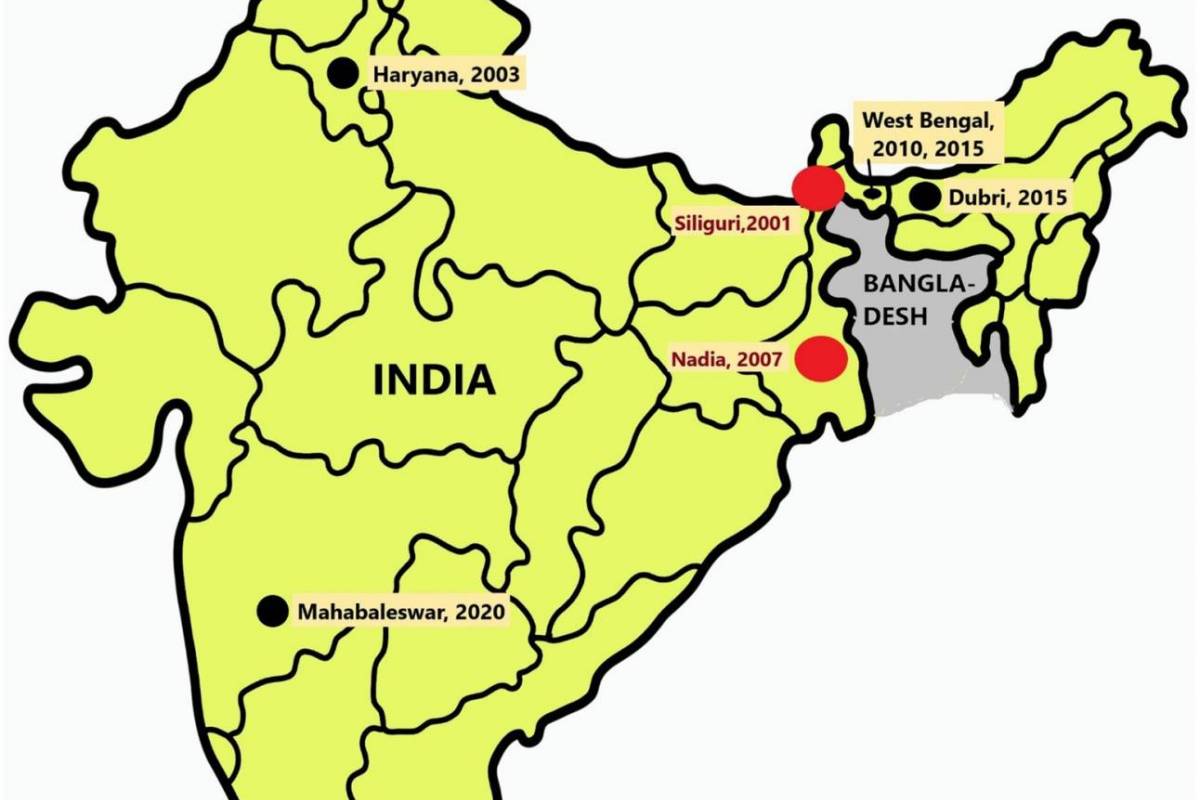
First identified after an outbreak in Malaysia, the Nipah virus causes small outbreaks in Bangladesh almost every year. In India, seven outbreaks of the Nipah virus have occurred since 2001, primarily occurring in the southern and eastern regions, according to a study published by the journal Frontiers.